Accurate simulation of aqueous-based electrochemical setups
Following the need for new and renewable sources of energy worldwide, fuel cells using electrocatalysts can be thought of as viable options. Catalyst materials modify and increase the rate of chemical reactions without itself undergoing any permanent change. An electrocatalyst is a catalyst that participates in electrochemical reactions and that functions at electrode surfaces or may be the electrode surface itself.
The interface between metal (the electrode) and water in these systems is the electrochemical central point, since it is the region where charge transfer can take place. A better understanding of the metal–water interface is also an essential requisite for predicting the correspondence between the macroscopic voltage and the microscopic interfacial charge distribution in electrochemical fuel cells. This reactivity is governed by the explicit atomic and electronic structures built at the interface as a response to external conditions, such as an applied potential.
The advance in experimental techniques for studying surfaces in the last decades started to provide important results concerning the local structure of water at interfaces, revealing a behaviour dependent on the reference electrical conditions set, the so-called bias. From a theoretical perspective this makes the task of simulating this setup difficult. In fact, accurate calculation of the electrostatic potential at electrically-biased metal–electrolyte interfaces is a challenge for ab initio simulations. Current methodologies attempt to simulate the effect of finite bias at the metal by altering their charge (adding/subtracting electrons), whereas in experiments the potential is the quantity that is controlled.
Now a team of researchers, including Pedro Brandimarte (CFM, DIPC), resolves 1 the local structure of water at the interfaces of metallic electrodes using a new computational method that allows to accurately simulate how atoms and molecules rearrange under an applied external potential using first principles methods, including ab initio molecular dynamics. This work opens the door to the accurate simulation of aqueous-based electrochemical setups.
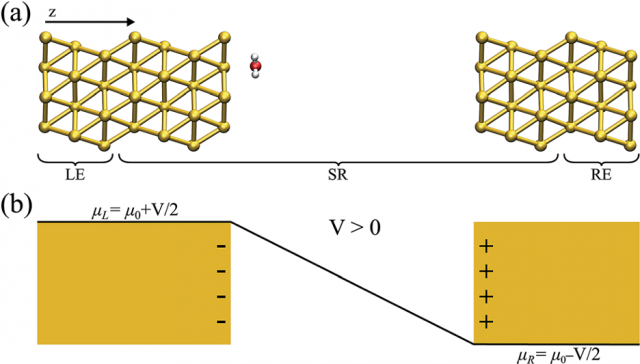
The electrochemical cell is usually thought of as two metallic electrodes which act as charge reservoirs, with the two metal plates separated by a solution (mostly composed of water). This arrangement is analogous to the one encountered in simulations of electronic transport: a central scattering region coupled to electrodes. And implies periodic boundary conditions.
In this new work, the researchers propose an alternative to this concept using open boundary conditions. This is done by employing the nonequilibrium Green’s function (NEGF) method that, as it name suggests, was designed to treat out-of-equilibrium situations, combined with density functional theory to properly compute the effect of an external bias potential applied to electrodes.
This combination has been developed over the past decade to describe the current–voltage characteristics of nanoscopic systems. It treats an open system under the influence of an external bias, and although dynamics – or forces – are typically ignored in such systems, they can be incorporated into the methodology.
The team applies this framework to a system consisting of a single water molecule between two Au(111) surfaces, at different configurations and as a function of an external voltage. This results in the successful description of a truly semi-infinite metallic electrode that sets a reference chemical potential that can be controlled by applying an external bias without adding/removing additional charge to the system. Meaning that a more direct comparison with the experimental setups is now possible as the electrochemical cell can be fully simulated, ionic currents and forces properly calculated and included.
This work opens the door to the accurate simulation of aqueous-based electrochemical setups.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance.
References
- Luana S. Pedroza, Pedro Brandimarte, Alexandre Reily Rocha and M.-V. Fernández-Serra (2017) Bias-dependent local structure of water molecules at a metallic interface Chemical Science doi: 10.1039/c7sc02208e ↩