Optimizing arrythmia ablation
The heart is a muscular organ with four chambers designed to work efficiently, reliably, and continuously over a lifetime. The muscular walls of each chamber contract in a regulated sequence, pumping blood as required by the body while expending as little energy as possible during each heartbeat.
Contraction of the muscle fibres in the heart is controlled by electricity that flows through the heart in a precise manner along distinct pathways at a controlled speed. The electrical current that begins each heartbeat originates in the heart’s pacemaker, located in the top of the upper right heart chamber (right atrium). The rate at which the pacemaker discharges the electrical current determines the heart rate. This rate is influenced by nerve impulses and by levels of certain hormones in the bloodstream.
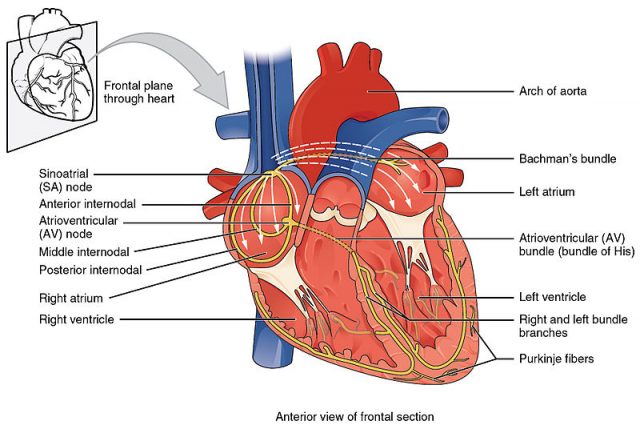
In other words, the normal heart beats in a regular, coordinated way because electrical impulses generated and spread by cells called myocytes with unique electrical properties trigger a sequence of organized myocardial (muscular) contractions. Arrhythmias and conduction disorders are caused by abnormalities in the generation or conduction of these electrical impulses or both.
Most arrhythmias neither cause symptoms nor interfere with the heart’s ability to pump blood. Thus, they usually pose little or no risk, although they can cause considerable anxiety if a person becomes aware of them. However, some arrhythmias, harmless in themselves, can lead to more serious arrhythmias. Any arrhythmia that impairs the heart’s ability to pump blood adequately is serious. 1
Therefore, the need for treatment varies; it is guided by symptoms and risks of the arrhythmia. Asymptomatic arrhythmias without serious risks do not require treatment even if they worsen. Symptomatic arrhythmias may require treatment to improve quality of life. Potentially life-threatening arrhythmias require treatment.
Treatment is directed at causes. Some possibilities include antiarrhythmic drugs, cardioversion-defibrillation, implantable cardioverter-defibrillators, pacemakers or surgery.
The cases where there is a problem with the conduction of the electrical impulses can be treated destroying the abnormal tissue that is interfering in the heart’s electrical system. Radiofrequency catheter ablation (RFCA) is a common and safe procedure to eliminate this tissue with heat.
In this procedure a radiofrequency current is delivered via an ablating electrode embedded at the tip of a percutaneous catheter and, as a consequence, the target tissue is burnt, meaning that the temperature reached is above 50ºC. There is a risk if the temperature in the electrode-tissue interface is significantly higher (around 80ºC). In this case, denaturation and adherence of blood proteins can occur with the possibility of the formation of a thrombus. In order to control temperature, thus avoiding the thrombus risk and simultaneously achieving larger thermal lesions, the so-called open-irrigated electrodes were developed. These are electrodes with built-in liquid refrigeration. They have small holes distributed around the electrode tip allowing the continuous flushing of a saline solution at the blood-tissue interface.
In order to optimize the procedure, computer modeling is the way to go. This approach allows to study different issues involved in RFCA in an efficient and reliable way, as opposed to ex vivo-in vitro and in vivo setups, which are time-consuming and lack reproducibility due to the several variables involved. To date, all previous models for endocardial RFCA mimicked the effect of the saline irrigation by simply fixing a constant temperature in the electrode tip, i.e. ignoring the saline motion problem. In fact, for a computer model of an open-irrigated electrode to be considered realistic it needs to account for both the saline irrigation flow and the blood flow motion. If both flows are not present, there is no way of obtaining a realistic distribution of the blood temperature in the cardiac chamber. As we have mentioned before, the blood temperature reached around the tip of the electrode is crucial since an excessive temperature directly leads to thrombus formation.
Now, a team of researchers, including Ana González-Suárez and Luca Gerardo-Gioca from BCAM, has set up the first computer model 2 for an open-irrigated electrode in endocardial RFCA which takes into account simultaneously the saline irrigation and the blood motion.
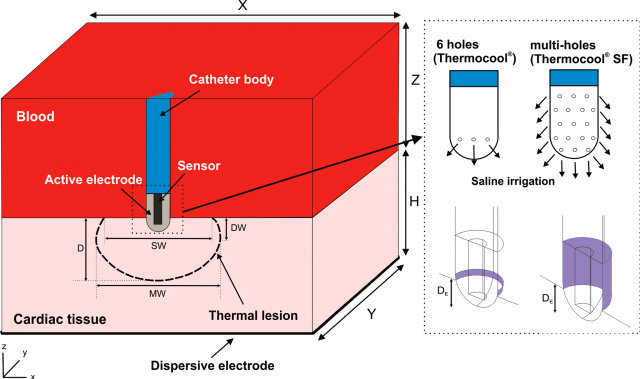
Once the model was built, the researchers conducted computer simulations under different conditions of irrigation flow rate, electrode-tissue contact and power settings. The thermal performance of the model was validated by comparing the computer results with previous experimental studies.
The simulation was able to adequately reproduce the findings of experimental studies, showing that the electrode design does not significantly affect the lesion volume and depth. As expected, the computer results also predicted that deeper and wider lesions are obtained in both perpendicular and parallel positions (the only ones considered) when a higher radiofrequency power is applied.
This model shows great potential in developing intervention strategies in terms of electrode design, tuning of the flow rate and the applied radiofrequency power to optimize arrhythmia ablation.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance.
References
- L. Brent Mitchell, Overview of Arrythmias, Merck Manual – Professional Version, revision September 2017 ↩
- González-Suárez A, Berjano E, Guerra JM, Gerardo-Giorda L (2016) Computational Modeling of Open-Irrigated Electrodes for Radiofrequency Cardiac Ablation Including Blood Motion-Saline Flow Interaction. PLoS ONE 11(3): e0150356. doi: 10.1371/journal.pone.0150356 ↩