Excited-state aromaticity
We usually take for granted that aromaticity is something that belongs to the ground state of a molecule; but the fact is that nothing forbids aromaticity appearing in an excited state. In the same way that molecular properties are largely affected by the ground-state aromaticity, excited-state aromaticity can guide the understanding of excited-state processes, the associated photochemistry, and, ultimately, will provide the tools for the development of novel functional materials.
Annulenes are organic hydrocarbons that have molecules containing simple single rings of carbon atoms linked by alternating single and double bonds. Such compounds have even numbers of carbon atoms. The simplest one is benzene, C6H6. Higher annulenes are usually referred to by the number of carbon atoms in the ring, as in [10]-annulene, C10H10.
You could expect that all annulenes are aromatic, like benzene, but that is not the case. Recall that aromaticity – the fact that all bonds are the same length intermediate between single and double C-C bonds – arise because the electrons in the π-orbitals are delocalized over the ring, giving an extra stabilization energy. The condition for such delocalization is that a compound should have a planar ring with (4n+2) pi electrons – this is known as the Hückel rule. Thus, [10]-annulene has (4n+2) pi electrons with n = 2, but is not aromatic because it is not planar. Aromaticity plays a key role in the understanding of molecular properties and chemical reactions due to its effects on the energy of the molecule.
A modification of the molecule usually results in changes in both the ground and the excited states. For revealing the effect of excited-state aromaticity, a modulation of aromaticity in the excited state is a prerequisite. Hence, in order to understand the aromaticity effects in the excited states, electronic effects that solely appear in the excited states without perturbing the ground-state nature are essential. Taking this into consideration, excited-state charge transfer processes, a shift of π-electron density between specific π-conjugated moieties in the excited states, can be a very effective approach to modulate the aromaticity only in the excited states.
Now, a team of researchers has focused 1 on an π-conjugated oligomer composed of a central 1,6-methano[10]annulene (M10A) and two 5-dicyanomethyl-thiophene (DT) peripheries in exo geometry. This compound (TMTQ) is an acceptor-donor-acceptor system, and the team has investigated a two-electron transfer process dominantly stabilized by an aromatization in the low-energy excited state.
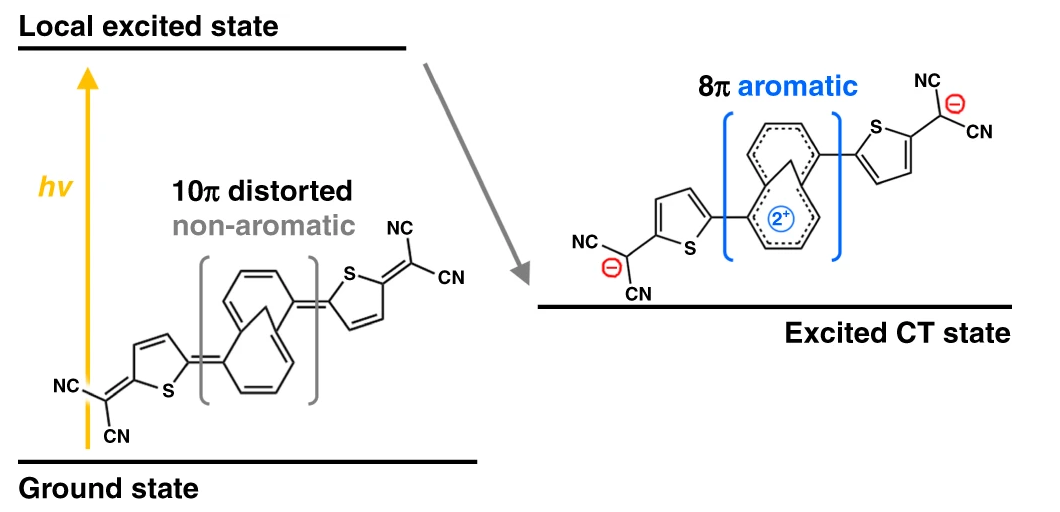
In TMTQ, the DT units serve as electron acceptors due to the strong electron-withdrawing nature of the dicyanomethylene groups. On the other hand, the non-aromatic core annulene of TMTQ, due to its highly distorted and quinoidal structure, facilitates M10A to act as an electron donor. This acceptor-donor-acceptor segmentation of TMTQ is an ideal configuration to promote excited-state intramolecular charge transfer processes.
Along the symmetric acceptor-donor-acceptor geometry, it is expected that the intramolecular charge transfer process gives rise to a shift of two π-electrons from M10A to DT units. Based on Baird’s rule – a reversal of ground-state aromaticity in the excited triplet state – this can lead to an aromatization of the core annulene in M10A by a change of its 10π electrons in the ground state into 8π electrons in the charge transfer (CT) state.
The authors demonstrate that the aromatization of TMTQ in the excited state is induced by the intramolecular charge transfer process, as it is directly and quantitatively observed using optical spectroscopic measurements. This suggests that the control and modulation of photoinduced charge-separation mechanisms can be one of the most effective approaches to control the excited-state aromaticity.
This observation gives a comprehensive understanding of the effect of excited-state aromaticity, which will provide crucial insights into the application of excited-state aromaticity for the design of functional photoactive materials.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research paper.
References
- Jinseok Kim, Juwon Oh, Seongchul Park, Jose L. Zafra, Justin R. De Francisco, David Casanova,Manho Lim, John D. Tovar, Juan Casado & Dongho Kim (2019) Two-electron transfer stabilized by excited-state aromatization Nature Communications doi: 10.1038/s41467-019-12986-w ↩