A path to systematically bridge the gap between molecular properties and solid-state material performance
Cromophores are generally groups of atoms having delocalized electrons. Covalent assemblies of conjugated organic chromophores provide the opportunity to engineer new excited states with novel properties. A team of researchers has now used a novel cage architecture 1 for creating them.
The properties of organic electronic materials being determined by the interactions between their conjugated subunits, one major challenge is understanding and controlling these interactions, which can give rise to new emergent properties of the assembly. In order to achieve that understanding, bulk solid-state samples, where disorder and the large number of interacting molecules make difficult a first-principles description of these new states, are not the best place to start. It is much easier to study smaller systems that can be purified and studied in isolation, for example, in dilute solution.
Even in these smaller systems the conformational flexibility of covalent assemblies can lead to multiple configurations that have different subunit interactions and, consequently, different electronic states and dynamics. Examples of such conjugated assemblies include donor-bridge-acceptor molecules, bichromophores and dendrimers. In these supermolecules, covalent linker groups define both the number and connectivity of the interacting conjugated subunits. In many cases, the conformational freedom of these covalent assemblies complicates their interpretation as structurally well-defined model systems.
A second problem is the role played by the covalent linker group in the electronic structure and dynamics of the assembly. Although we would like to assume that the linker is inert, the truth is that a large body of work says otherwise, showing that it can play an important role in facilitating charge and energy transfer.
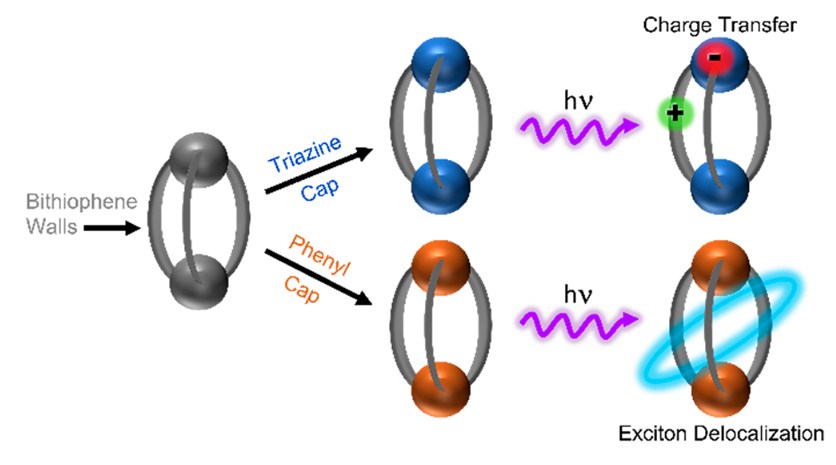
Any new architecture that would limit conformational freedom and help establish the actual role of the linker would be, thus, most welcome. This is exactly what the new work proposes. Meta-substituted aromatic caps serve as the covalent linking groups that attach to both top and bottom of the conjugated subunit. This strategy enables improved control of both molecular spacing and orientation because it provides two points of attachment for each conjugated subunit, bringing the assembly closer to crystalline order. Furthermore, the capping group acts as a constant structural element while providing chemical tunability that can be used to actively modify the electronic
structure of the assembly.
To demonstrate the capabilities of the architecture shown in the researchers study the properties of a bithiophene trimer built as a cage with two different aromatic capping units. A simple phenyl cap is an example of a passive capping group whose electronic states do not mix with those of the chromophore walls. The excited state properties are dominated by through-space interactions between the chromophore subunits themselves. A triazine cap, on the other hand, provides the opportunity for chromophore-linker electron transfer and leads to the formation of new states with enhanced fluorescence.
The ability to create different nanoscale heterostructures while retaining the overall morphology provides an unprecedented opportunity to tune the properties of these discrete assemblies. These results provide a new route toward structurally well-defined multichromophoric assemblies whose excited states can be rationally designed using the tools of organic synthesis and computational chemistry. As this architecture is in principle scalable, it may provide a path to systematically bridge the gap between molecular properties and solid-state material performance.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research paper/s.
References
- Taylor N. Lewis, Claire Tonnelé, William G. Shuler, Zachary A. Kasun, Hiroki Sato, Adam J. Berges, Jacob R. Rodriguez, Michael J. Krische, David Casanova, and Christopher J. Bardeen (2021) Chemical Tuning of Exciton versus Charge-Transfer Excited States in Conformationally Restricted Arylene Cages JACS doi: 10.1021/jacs.1c08176 ↩