A Tale of Primary Cilia: from overlooked organelles to key mechanically-sensing antennae
Author: Jose V. Torres-Perez (@Jovitope) is a Postdoctoral Researcher at the School of Biological and Chemical Sciencies, Queen Mary, University of London (UK).
This is the story of primary cilium, a cellular structure discovered at least 122 years ago. It was soon regarded as rudimentary and then forgotten for most researchers. Nonetheless, recent advances proved its role as a cellular sensor and its implications in multiple pathologies. Since then, it has become a hot topic for biomedical research. The advances on primary cilia’s research mirror those of high-resolution microscopy in a tale that reflex the importance of technical advances in the discovery of the natural world and show the importance of interdisciplinary research. It was in this context that I, a neuroscientist, ventured into an engineering department to study the role primary cilia have on mechanotransduction, cells’ ability to perceive mechanical alterations such as flow, pocking, touching or pinching. This investigation, supervised by Dr Pavel Novak at Queen Mary University of London, has resulted in a recently published article at Cellular Physiology & Biochemistry.
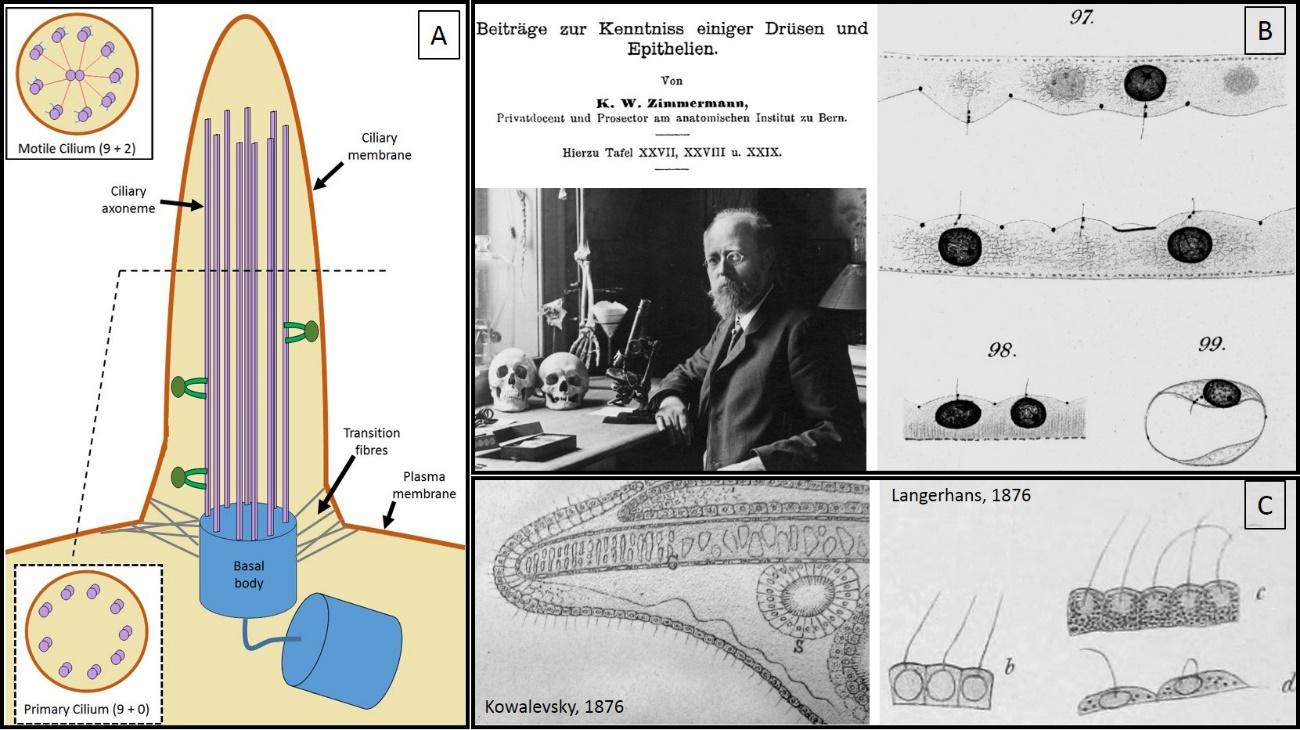
Primary cilia (figure 1) are small non-motile cylindrical cellular protrusions. When seen through a microscope, they look like small hairs that extend beyond the cells. In fact, their name (cilium in singular, cilia in plural) derives from the Latin for “eyelashes”. They are present in most types of cells in our body such as muscular, endothelial, connective, epithelial or stem cell types, and even neurons. Primary cilia form a separate cellular compartment from the rest of the cell and have a specific population of proteins within them, which confers them special properties.
Nowadays, we know that lack of cilia, or when they don’t work right, leads to different pathologies such as polycystic kidney disease (PKD), a genetic disorder in which kidneys develop multiple cysts (renal tubules that fill with liquid and stop working). Not surprisingly, the term ciliopathies have emerged in recent years to group all those pathological disorders in which cilia are affected.
In opposition to the motile cilia (see figure 1.A), their close cellular relatives, primary cilia lack the central pair of microtubules (structural proteins that help support their shape and function) which accounts for their inability to actively move. Further, while different cell types can have several motile cilia, only one single primary cilium (if any) is present in each cell. In fact, it is the lack of motility and the “residual” presence that made past researchers consider primary cilia as evolutionary vestigial structures with non-significant functions that have been spared from disappearing by natural selection.
As with many other biological phenomena, cilia research has nourished from advances in laboratory techniques and multidisciplinary research that made possible to visualise and comprehend them. Their discovery had to wait until the development of the first microscopes by Antoni van Leeuwenhoek (late 17th century). However, initial focus was only placed on the motility of cilia, the sole function attributed to these tinny hair-like cellular protrusion.
It was not until late 19th century that primary cilia got separated from the motile close-relatives and established themselves as a completely distinct class of cilia. This was mainly due to the discovery and characterization of the centriole, a key cellular component for cell division, and, surprisingly, for the generation of primary cilium’s basal body. Karl Wilhelm Zimmermann (see figure 1.D), a German anatomist and histologist, was the first one to fully describe them in 1898. Although not the first one to observe them (see figure 1.C), he was the first one to identify them as different organelles and named them “centralgeissel” (central flagella).
As time passed by, primary cilia were thought to lack any specific function and most of the scientific community lost interest on them. However, at the 90s (see figure 2.A), different exciting research papers proved their key role to sense, integrate and convey different kinds of information from the outside environment to the core of the cell, hence gaining them the nickname of cellular antennae. For instance, in 2003, Helle A. Praetorius and Kenneth R. Springs showed that primary cilia are of vital importance to sense mechanical alteration in kidney epithelial cells. They act as sensors to any kind of movement by extending to the light of kidney’s tubes, in the same way that a kite flying up in the sky can catch wind alterations and transfer this information (which could not have been sensed otherwise) to us on the ground.
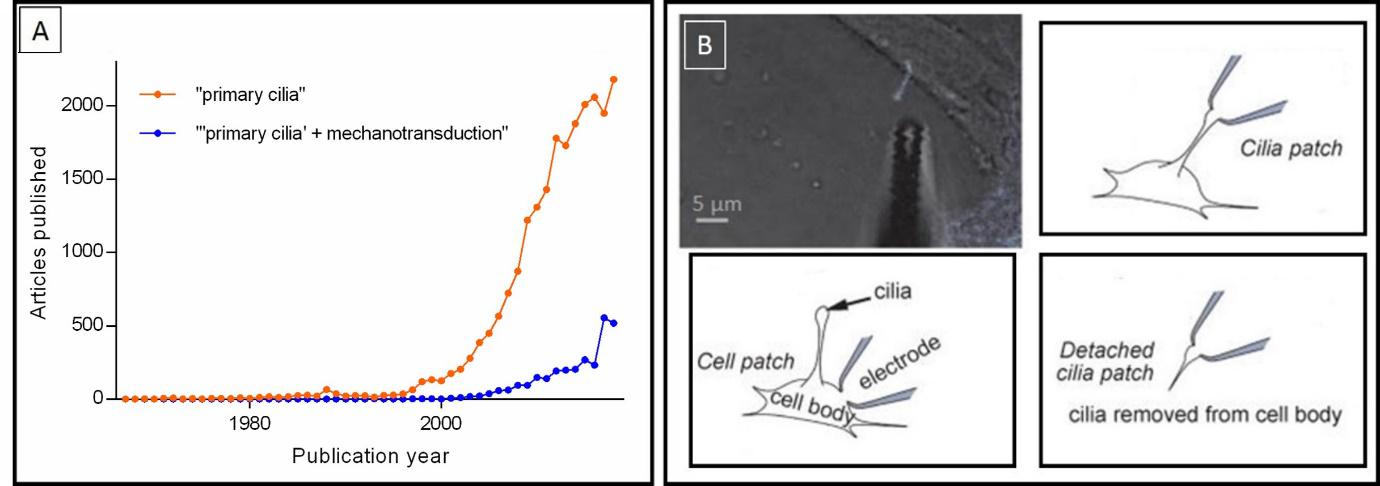
However, the whole process by which primary cilia convey information to the rest of the cell is still not fully understood. Some scientific articles suggest that internal releases of calcium act as the “molecular currency”, while others point at ATP. However, novel research describes the lack of mechanically induced calcium signals in the cilia, thus contradicting previous findings. These incongruences on present data are partly a result of the technical difficulties to establish direct measurements from the fine structure of primary cilia: their diameter is below the resolution limit of optical microscopy, which accounts as an extra difficulty when attempting to record responses directly from these organelles.
As a milestone in the field, Dr David E. Clapham’s group, from Harvard University, performed what could be considered as the last frontier on primary cilia’s research: the first-ever direct electrophysiological recordings (a measure of the electrical activity) from primary cilia (see figure 2.B). In their experiments, they successfully recorded directly from the tip of cilia either attached or detached from the cell and observed striking differences in the population of receptors. Although extremely interesting and challenging, their research presented few limitations: suction was needed to place the cilia in the recording apparatus (the pipette) and they could not specify the recording location within the cilia.
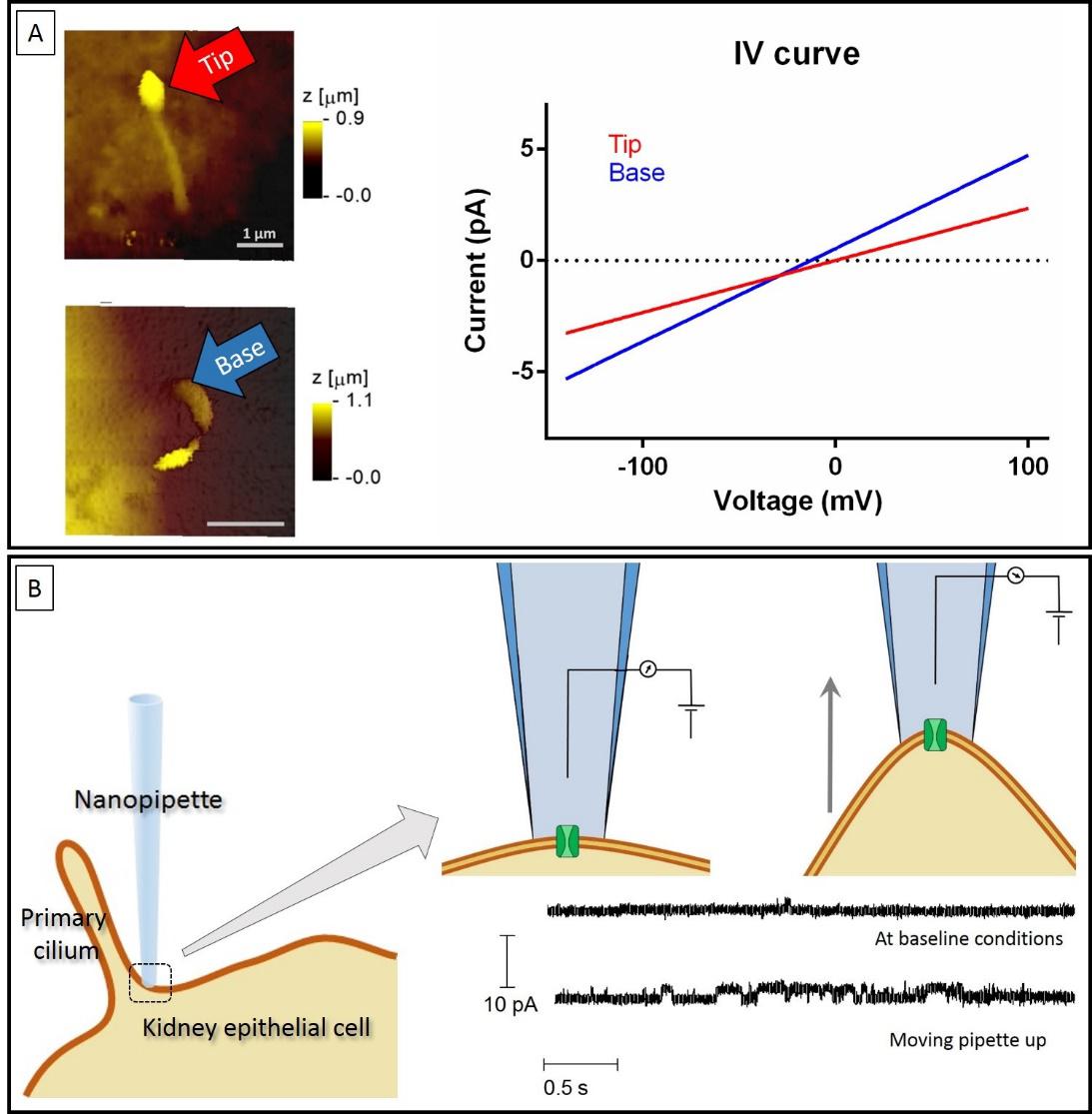
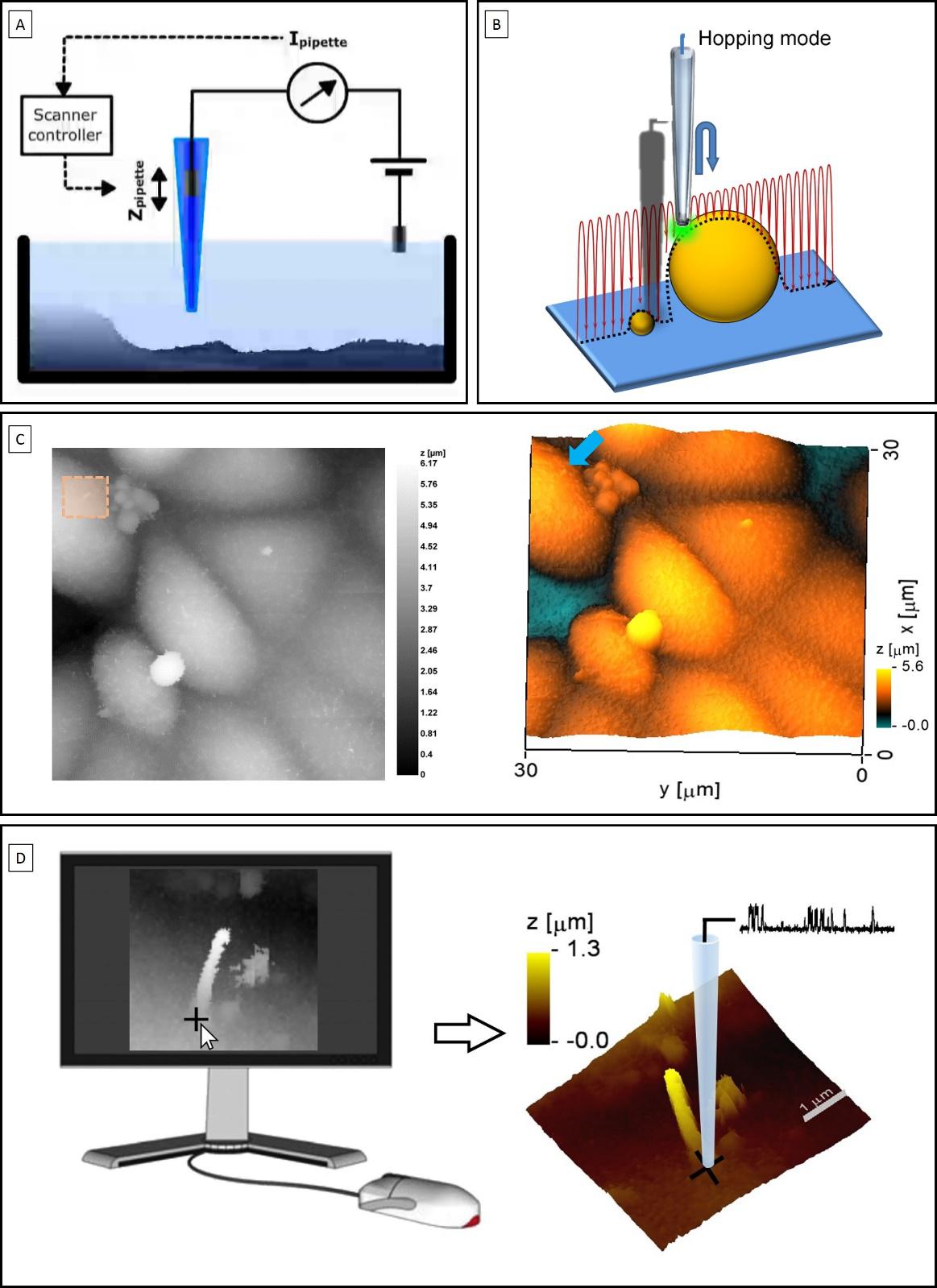
Finally, with the research that I am presenting here 1, we managed to move one step forward by directly recording from different ciliary portions within kidney epithelial cells. To do so, we combined Scanning Ion Conductance Microscopy (SICM) with electrophysiology, which is referred as smart-patch clamping. SICM is a type of microscopy that uses a glass pipette as a probe to scan the surface of different substrates, including living cells (see figure 3.A). With the hopping variant of this method we obtained topographical images of primary cilia at a nanoscale resolution (see figure 3.B and 3.C). Once a cilium was identified, same probe was used to perform electrophysiological recordings by simply positioning the pipette at a location selected on the topographical image (see figure 3.D). Using this technology, we successfully obtained recordings from the base and the tip of cilia where we observed a differential distribution of receptors (see figure 4.A).
Furthermore, we used this setup to do an initial characterization of mechano-sensitivity at these specific locations along the cilium: once a recording was established, we used precisely controlled displacement of the pipette, which then also dragged the patch of membrane attached to it, to assess differences in activity. Strikingly, we observe that there was a massive increase in activity induced by the displacement near the base of cilia, while the tip responded differently (see figure 4.B).
Taking all together, our experiments are the first to show local variations in the sensitivity to mechanical alterations in kidney epithelial cells by combining SICM with electrophysiology. They also show that the base of primary cilia seems to play a key role to transduce mechanical stimulations to the core of the cell. Going back to the analogy of the kite: our research points at the base of the threat, the place where the kite connects with the ground, as the key portion in which mechanical alterations are translated into meaningful signals of the movements happening in the sky. In there, the anchorage of this threat to the ground seems to generate a maximal tension, strong enough to even detect the smaller alterations happening higher up.
Moving forward, this setup could be combined with genetic strategies or with the use of specific drugs to fully characterise the channels responsible for mechanotransduction in this cell type as well as in many others. The significance of this study opens a new window in primary cilia’s research that could not have been assessed with conventional recording techniques and highlights the importance of new analytical strategies and the need to work in multidisciplinary teams if we aim to gain a full meaning of the biological world.
BIBLIOGRAPHY:
-
Decaen, P. G.; Delling, M.; Vien, T. N.; Clapham, D. E. Nature 2013, 504, 315–318.
-
Delling, M.; Indzhykulian, A.A.;Liu, X.; Li, Y.; Xie, T.; D.P. Corey, D.P.; D.E. Clapham, D.E. Nature 2016, 531, 656-660.
-
Novak, P.; Li, C.; Shevchuk, A. I.; Stepanyan, R.; Caldwell, M.; Hughes, S.; Smart, T. G.; Gorelik, J.; Ostanin, V. P.; Moss, G. W. J.; Frolenkov, G. I.; Klenerman, D.; Korchev, Y. E.; Lab, M. J. Nat. Methods 2009, 6, 279–281.
-
Praetorius, H. a.; Spring, K. R. J. Membr. Biol. 2003, 191, 69–76.
-
Praetorius, H.A.; Spring, K.R. J Membr Biol 2001, 184, 71-79.
-
Sloboda, R.D. Methods in Cell Biology 2009, 94, 1-386.
-
Wann, A.K.; Zuo, N.; Haycraft, C.J.; Jensen, C.G.; Poole, C.A.; McGlashan, S.R.; Knight, M.M. FASEB J 2012, 26, 1663-1671.
References
- Torres-Pérez, J.T.; Naeem, H.; Thompson, C.L.; Knight, M.M.; Novak, P. Nanoscale mapping reveals functional differences in ion channels populating the membrane of primary cilia. Cell Physiol Biochem 2020, 54, 15-26. doi: 10.33594/000000202 ↩
2 comments
[…] Pertsonentzat ilea dena dira zilioak zelulentzat. Zerbaiterako balio dutela suposatzen da, horretarako baitaude, baina garrantziarik gabeko kontua izango da. Zilioen kasuan, ikerketa sakon batek gauza harrigarriak ekarri ditu argira: José V. Torres-Pérezren A Tale of Primary Cilia: from overlooked […]
[…] Los cilios son a las células lo que el pelo a las personas, o poco más o menos. Se suponen que sirven para alguna cosa, porque por eso están ahí, pero será una cosa poco importante. Al menos en […]