Controlling single molecule conductance with a new class of covalent bond formation
Controlling single molecule conductance with a new class of covalent bond formation
One of the greatest inventions of the 20th century, if not the greatest, was the transistor. It revolutionized the electronics industry and changed the way people around the world lived, learned, worked, and played. Its invention marked the beginning of solid state electronics which quickly reduced the size and power requirements of existing electronic tube based electronic devices. This revolutionized the electronics field and eventually made possible the information age thanks to small, low power electronic devices and eventually low cost integrated circuits.
Now, in the 21st century, research is focusing on achieving what the transistor did but using just a few molecules or, even, only one. These molecules may very well be organic ones: we are entering the era of organic electronics. The emerging devices include organic light-emitting diodes, organic solar cells and organic transistors, to name just a few.
A key point in the development of these devices is how can we connect and control these molecules with the rest of the “electronics” of the device. The problem is even bigger when we are using only one molecule. A better understanding of the electronic properties at the individual molecular level and the development of methods to tune the charge transport through molecular junctions is, therefore, a prerequisite.
One of the challenges associated with reducing electronic circuits to single-molecule components is the formation of reliable, high-conductance contacts between the molecule and the metallic electrodes to enable an efficient charge transport across the metal–organic interface.
The conductance through a single molecule can be measured by contacting the molecule with atomic precision and forming a molecular bridge between a metallic scanning tunneling microscopy tip electrode and the metallic surface electrode where the molecule has been placed. But, can it also be controlled?
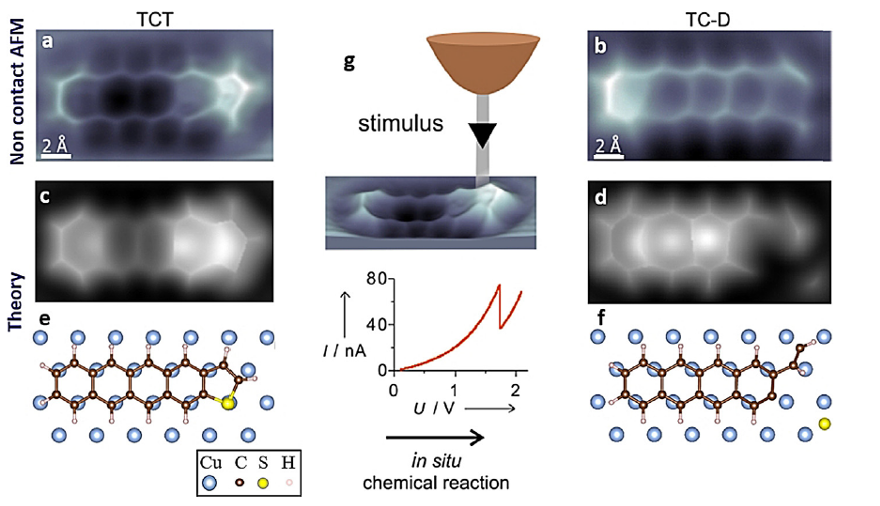
Now, a team of researchers shows 1 that an in situ induced direct chemical reaction strongly improves the molecular anchoring by forming covalent bonds between molecular carbon and metallic surface atoms, and that this bond formation leads to an increase of the conductance by about 50% compared to the initial state.
While π-conjugated systems with a delocalized electronic structure typically have a high electronic conductance through the molecules, they usually present non-conjugated end groups designed to selectively couple the molecules to specific metallic electrodes. Covalent bonds between the molecule and the metal electrodes are advantageous to ensure a robust mechanical and electronic connection. In the ideal case, the organo–metallic contacts of the molecular electronic element are established through a chemical reaction between the molecule and the metal. The most popular examples are systems with covalent bonds formed between sulfur-containing molecular end groups and gold electrodes.When the anchoring is done through direct covalent bonds between the carbon atoms and the metal, a higher electronic conductance is obtained.
The scientists build on a previous result, where they showed an in situ induced direct desulfurization reaction on single thiophene units on Cu(111) surfaces driven by the electric field confined in the tunnel junction. The researchers now explore the molecule-metal-electrode anchoring and the conductance across a tetracenothiophene molecule, a pentacene analogue, by controlling the formation of covalent C-Cu bonds between the molecule and the metal substrate on the submolecular scale. This is a new class of covalent bond formation between a chemically reacted thiophene molecular end group and Cu electrodes.
They find that the resulting tetraceno derivative shows a significant increase in the conductance, as a result of a better contact formation between the bifurcated end of the remaining thiophene part and the metal surface, whose fingerprint is an intramolecular enhancement of the local density of states close to the Fermi energy.
This is the first step of a new approach for an efficient molecule-metal-electrode anchoring and a drastic increase in conductance essential for single-molecule electronic device elements.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may be copied verbatim or almost verbatim from the referenced research paper.
References
- Tomasz Michnowicz, Bogdana Borca, Rémi Pétuya, Verena Schendel, Marcel Pristl, Ivan Pentegov, Ulrike Kraft, Hagen Klauk, Peter Wahl, Pingo Mutombo, Pavel Jelínek, Andrés Arnau, Uta Schlickum, and Klaus Kern (2020) Controlling Single Molecule Conductance by a Locally Induced Chemical Reaction on Individual Thiophene Units Angew. Chem. Int. Ed. doi: 10.1002/anie.201915200 ↩