Cutaneous lymphocyte antigen is a potential therapeutic target in cutaneous T-cell lymphoma
Cutaneous
Author: Marta Irigoyen is a postdoctoral researcher at CIC bioGUNE
Cutaneous T-cell lymphoma (CTCL) is a rare type of cancer that begins in white blood cells called T cells (T lymphocytes). These cells normally help the body’s immune system to fight germs and pathogens. In cutaneous T-cell lymphoma, T cells acquire aberrant functions that cause them to attack the skin. The most common form, mycosis fungoides (MF), is characterized by an indolent evolution with patches and plaques on the skin and atypical clonal T cells that colonize the epidermis (the top layer of the skin). In contrast, the more aggressive form known as “Sézary syndrome” (SS), is less frequent and characterized by a skin inflammation associated with pruritus and a clonal expansion of T cells circulating in the blood 1. Unfortunately, patients with SS have a poorer prognosis than those with MF, with a 5-year survival rate for the 24‒40% of individuals 23. Treatments are not curative, especially in advanced stages, except in cases eligible for stem cell transplantation 4.
Recent studies have focused on the immunophenotypic characterization of CTCL using flow cytometry and have shown that skin biopsies of MF and SS contain skin-infiltrating lymphocytes expressing cutaneous lymphocyte antigen (CLA) in the vast majority of cases 5. Although CLA expression by CTCL cells has been reported, the role of CLA molecule in CTCLs is unknown. In this work, Sara Peru and collaborators decided to evaluate CLA functions on migration and survival of patient SCs and a MF cell line (My-La cell line) 6.
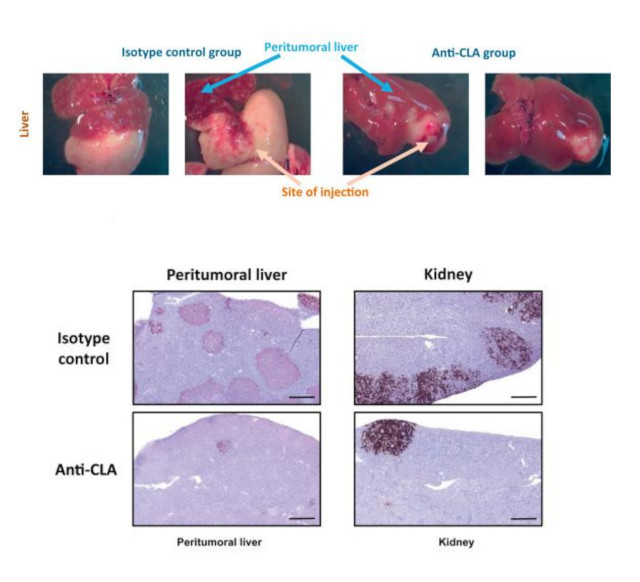
Above: pictures show a decrease in tumor size at the site of injection (liver) in the anti-CLA antibody group compared with that in the control group. Lower sections show a decrease of CTCL cells in the peritumoral liver and the kidney of mice injected with cells treated with anti-CLA mAb compared to mice treated with the isotype control. Image modified from Peru, S. et al. J Invest Dermatol (2022) DOI: 10.1016/j.jid.2022.06.016.
Firstly, authors evaluated by flow cytometry the percentage of CLA+ T cells in 36 patients with SS and they showed that the percentage of CLA+ SCs was heterogeneous among patients: a total 34 of the 36 patient samples exhibited more than 10% of CLA+ cells, and 16 exhibited more than 50% of CLA+ cells in the tumor clonal population.
It has been shown that T cells expressing CLA interact with the E-selectin, an adhesion molecule expressed by activated endothelial cells 7, and that interaction is necessary for the skin-homing properties of T cells. Thus, the authors investigate the involvement of CLA in CTCL cell migration by targeting CLA with and antibody (anti-CLA mAb) in a transendothelial migration assay using a Transwell system coated with human endothelial cells. The percentages of migrating My-La cells and SCs treated with an anti-CLA mAb were significantly lower than that of the control antibody group. In parallel, they also increased the expression of CLA ligand (E-selectin) on endothelial cell surface by mimicking the inflamed tissue by lipopolysaccharide exposure. My-La cell migration was also significantly increased after E-selectin induction and impaired after anti-CLA mAb treatment. Interestingly, in an inflammatory context, anti-CLA antibody had higher effect in the inhibition of cell migration than the steady state condition. These data showed that CLA is involved in CTCL migration process through endothelial cells.
To investigate whether CLA participates in CTCL cell survival, they performed an apoptosis assay on the My-La cell line and on fresh CLA+ SCs from patients after treatment with anti-CLA mAb. They showed that the percentage of total cells death was significantly increased when cells were treated with the anti-CLA mAb in comparison with the control antibody treatment. Hence, these data supported that CLA expression is involved in CTCL survival.
The CLA molecule shares a common core with PSGL-1 (a glycoprotein found on the surface of white blood and endothelial cells that binds to P-selectin) and differs by post-translational modification mediated by the enzyme ST3Gal-4 (the maturation enzyme of CLA). To confirm whether the decrease of CLA levels could affect cell survival, they silenced ST3Gal-4 using shRNA metodology. Thus, ST3Gal-4 inhibition was associated with a significant decrease in trans-endothelial migration capacity of CTCL cells. The decrease of CLA expression at the surface of CTCL cells was also associated with significantly increased cell death and a reduction of total cell numbers in vitro. These data supported that the presence of CLA on the cell surface is involved in CTCL trans-endothelial migration, proliferation and survival.
To understand the mechanisms triggering cell death induced by the anti-CLA mAb, authors measured the activity of caspases-3/7, caspase-8, and caspase-9 and mitochondria permeability in My-La cells. They observed that caspases 3/7, caspase-8 and caspase-9 were increased by the anti-CLA mAb. Besides, Tetramethylrhodamine methyl ester (a fluorescent dye sequestered by active mitochondria) staining was also reduced after treatment with the anti-CLA mAb, showing a loss of the mitochondrial membrane potential. During apoptosis, an increase in the permeability of the mitochondrial membrane is crucial and results in the release of several apoptogenic factors into the cytoplasm. Altogether, the anti-CLA antibody induced rapid activation of the different apoptotic pathways in My-La cells.
Finally, to test whether anti-CLA treatment was able to impair the tumourigenic capacity, My-La cells were previously treated with the anti-CLA mAb or with the corresponding control antibody and then injected in immunodeficient mice. After 2 weeks of clinical monitoring, the mice were killed and macroscopic analysis showed a decrease in tumor size at the site of injection for the anti-CLA antibody group respect to the control group. Remarkably, the percentage of My-La cells on peritumoral liver and kidney sections was significantly lower in the anti-CLA group than in the control group. Thus, the anti-CLA treatment on My-La cells led to a decrease in both tumorigenesis and the spreading of My-La cells in different tissues.
In summary, using the in vivo approach, this work confirmed that treatment with anti-CLA mAb was able to decrease the tumorigenicity as well as the dissemination of CTCL cells in different tissues. Besides, the findings provide evidence of the involvement if CLA in CTCL migration and survival, supporting that CLA inhibition could represent a promising therapy in patients with CTCL.
References
- Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood 2007; 110: 1713-1722. PMID: 17540844 DOI 10.1182/blood-2007-03-055749. ↩
- Fink-Puches R, Zenahlik P, Bäck B, Smolle J, Kerl H, Cerroni L. Primary cutaneous lymphomas: applicability of current classification schemes (European Organization for Research and Treatment of Cancer, World Health Organization) based on clinicopathologic features observed in a large group of patients. Blood 2002; 99: 800-805. PMID: 11806979 DOI: 10.1182/blood.v99.3.800. ↩
- Wilcox RA Cutaneous T-cell lymphoma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol 2016; 91: 151-165. PMID: 26607183. DOI: 10.1002/ajh.24233. ↩
- de Masson A, Beylot-Barry M, Bouaziz JD, Peffault de Latour RP, Aubin F, Garciaz S et al. Allogeneic stem cell transplantation for advanced cutaneous T-cell lymphomas: a study from the French Society of Bone Marrow Transplantation and French Study Group on Cutaneous Lymphomas. Haematologica 2014; 99: 527-534. PMID: 24213148 DOI: 10.3324/haematol.2013.098145. ↩
- Kamarashev J, Burg G, Kempf W, Hess Schmid MH, Dummer R. Comparative analysis of histological and immunohistological features in mycosis fungoides and Sezary syndrome. J Cutan Pathol. 1998; 25: 407-412. PMID: 9826165 DOI: 10.1111/j.1600-0560.1998.tb01766.x. ↩
- Peru S, Prochazkova-Carlotti M, Cherrier F, Velazquez J, Richard E, Idrissi Y et al. Cutaneous Lymphocyte Antigen Is a Potential Therapeutic Target in Cutaneous T-Cell Lymphoma. J Investo Dermatol 2022; 142: 3243-3252. PMID: 35850209 DOI: 10.1016/j.jid.2022.06.016. ↩
- Fuhlbrigge RC, Kieffer JD, Armerding D, Kupper TS. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature 1997; 389: 978-981. PMID: 9353122 DOI: 10.1038/40166. ↩