p21 is a target for phagocytosis-mediated cellular immunotherapy in acute leukemia
Phagocytosis
Author: Marta Irigoyen is a postdoctoral researcher at CIC bioGUNE
Phagocytosis of cancer cells by tumor-associated macrophages (TAMs), which are abundant in the tumor microenvironment (TME), plays a critical role in cancer immunosurveillance 1. Cancer cells can evade macrophage-mediated phagocytosis (the process by which a cell uses its plasma membrane to engulf particles or other cells) by upregulating cell-surface expression of “don’t eat me signals”. Several immunotherapies aim to disrupt these interactions through the use of macrophage immune checkpoint (MIC) blockers to escape signaling that inhibits phagocytosis, enabling macrophages to engulf and clear cancer cells 23.
In addition to the impairment in their phagocytic functionalities, TAMs demonstrate an immunosuppressive phenotype that contributes to tumor progression, treatment resistance, and poor clinical outcomes 4. Several therapeutic approaches targeting TAMs to mitigate their immunosuppressive properties or promote their phagocytic capacities to engulf tumor cells have shown therapeutic benefits in several tumor types 5, but therapeutic strategies aimed at promoting both the tumor cell phagocytosis and proinflammatory reprogramming of TAMs are rarely considered as therapeutic options for cancer treatment.
T-ALL is an aggressive malignancy which results from the leukemic transformation of T-cell progenitors (the cells responsible to generate the white blood cells called lymphocytes T) into tumor cells. Despite the tremendous progress in T-ALL therapies during the last decades, an elevate percentage of patients shows resistance and the fear some tumor relapse.
Due to the high resistance to conventional therapies shown in patients with T-ALL, and taking into account the potential role of targeting the phagocytic capacities of TAMs as a promising therapeutic opportunity, Awatef Allouch and collaborators aimed to improve the understanding of the interplay between the abrogation of tumor cell phagocytosis resistance and the pro-inflammatory functional reprogramming of TAMs to fight T-ALL 6.
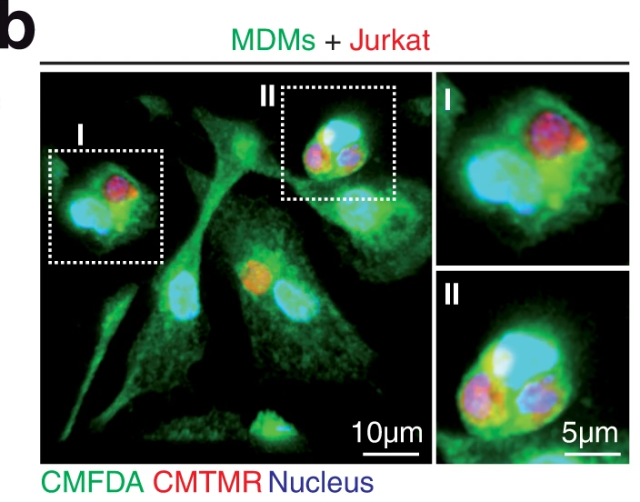
To decipher the interplay between the abrogation of tumor cell phagocytosis resistance mechanisms and the proinflammatory activation of macrophages, authors first of all aimed to analyzed if human blood monocyte-derived macrophages (MDMs) were able to phagocyte T-ALL cells. As expected, when MDMs were cocultured with different human leukemia cell lines MDMs rapidly engulfed T-ALL cells and delivered them to lysosomal compartments for degradation.
To explore the functional impact of leukemia cell phagocytosis on the MDM phenotype, they cultured MDMs with a T-ALL cell line and then separated phagocytic (Phago+ MDMs) and non–phagocytic (Phago− MDMs) cells. Phago+ MDMs showed a complete degradation of engulfed cells and gene expression analysis indicated that after target cell degradation, Phago+ MDMs underwent pro-inflammatory activation, as indicated by the regulation of different genes, highlighting Interferon γ (IFNγ). Since this molecule, which is secreted by Phago+ MDMs, could convert immunosuppressive TAMs into immunostimulatory macrophages 7, they next explored the ability of secreted IFNγ to promote the pro-inflammatory reprogramming of surrounding Phago- MDMs. Interestingly, they corroborated that Phago+ MDMs could polarize anti-inflammatory MDMs into a pro-inflammatory phenotype through the secretion of IFNγ. Considering (i) the shared cellular features between macrophages and senescent cells (senescent is a cellular state in which cells no longer have the ability to divide but are still alive and metabolically active) 8 , (ii) the ability of senescent cells to phagocytose live tumor cells 9, and (iii) the pivotal roles of p21 in the senescence 10, terminal differentiation, and survival of macrophages 11 12, authors assessed the role of p21 to identify molecular mechanisms regulating the macrophage-mediated phagocytosis of leukemia cells. Then, they inhibit the expression of p21 in MDMs and analyze for phagocytic activity. Interestingly, p21 depletion strongly reduced the phagocytosis of different T-ALL cell lines. Importantly, the exogenous expression of p21 in p21-depleted MDMs restored the phagocytic activity of these MDMs, further corroborating the specific key role of p21 in promoting the phagocytosis of leukemia cells by macrophages.
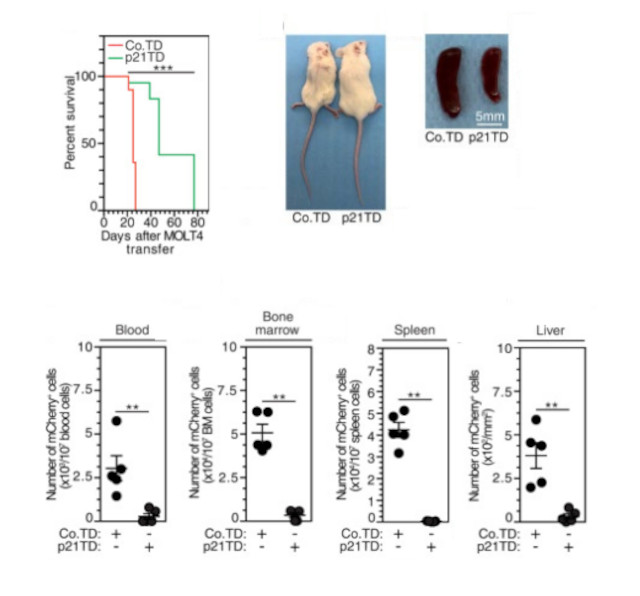
To explore how the p21-mediated modulation of MDM phagocytic activity toward leukemia cells could be used therapeutically, authors firstly verified that when they transfer human monocytes (mos) into immunosuprissed (NSG) mice they differentiate into macrophages. Then, they generated monocyted which express p21 (p21TD-Mos) and confirmed that they diferentiated into macrophages when they are transferred to NSG mice. Therefore, they transferred modified p21TD-Mos into NSG engrafted with MOLT4 cells (a human T-ALL cell line) and they showed a significant reduction in the leukemic burdens in the peripheral blood, bone marrow, spleen and liver of p21TD-Mo-treated mice compared with those in the same tissues of mice treated with normal monocytes. Consistently, a significant increase in survival was also observed in p21TD-Mo-treated leukemia mice. Altogether, these results demonstrated that the transfer of p21TD-Mos strongly reduced the progression of leukemia.
Given the key role of IFNγ secretion by Phago+ MDMs during in vitro pro-inflammatory reprogramming of surrounding Phago- MDMs, authors decided to determine the contribution of this pro-inflammatory cytokine to the antitumor effect promoted by the adoptive transfer of p21-generated Mos. Specifically, p21TD-Mo-transferred NSG mice engrafted with MOLT4 cells were treated with anti-human IFNγ (hIFNγ) blocking antibodies 15 days after Mos transfer and analyzed for survival. Treatment with the blocking antibodies strongly reversed the increase in survival observed for mice that received p21TD-Mos. These data confirmed that IFNγ secreted by phagocytic p21-engineered TAMs in response to the pro-inflammatory reprogramming of surrounding TAMs is a central cytokine in the antitumor effect detected after Mos transfer. Altogether, these results indicate that p21TD-Mo-based cellular therapy drives the engraftment of p21-modified phagocytes, which, in addition to directing the elimination of leukemia cells, triggers the secretion of the pro-inflammatory cytokine IFNγ and supports the pro-inflammatory reprogramming of TAMs, which in turn participate in the regression of leukemia.
To further explore the therapeutic effect of p21TD-Mo-based cellular therapy, they treated NSG mice engrafted with patient-derived T-ALL cells, including two Patient-derived xenograft (PDX) models (in this model, T-ALL cells from patients are implanted into NSG mice), derived from the same patient at diagnosis (PDX#1) and at relapse (PDX#2). Transfer of p21TD-Mos significantly increased the survival of T-ALL PDX-engrafted mice compared with adoptive transfer of unmodified Mos. Moreover, the p21TD-Mo-based cellular therapy remained effective in mice engrafted with T-ALL cells at relapse after one year of chemotherapy. Altogether, these results support the translational potential of the therapeutic induction of p21-mediated tumor cell phagocytosis for the treatment of T-ALL.
In summary, adoptive transfer of p21TD-Mos would represent a broad-spectrum therapy for leukemia because it can enhance the macrophage capacity for phagocytosis without affecting antigen pressure selection, which could induce the emergence of clonal resistance 13. Finally, considering the current difficulties associated with eradicating acute leukemia, adoptive transfer of p21TD-Mos should be considered a strategy that could complement commonly used chemotherapeutic approaches.
References
- Chao MP, Majeti R, Weissman IL. Programmed cell removal: a new obstacle in the road to developing cancer. Nat Rev Cancer 2011; 12: 58-67. PMID: 22158022 DOI: 10.1038/nrc3171. ↩
- Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 2009; 138: 271-285. PMID: 19632178 DOI: 10.1016/j.cell.2009.05.046. ↩
- Weiskopf K, Ring AM, Ho CCM, Volkmer JP, Levin AM, Volkmer AK et al. Engineered SIRPalpha variants as immunotherapeutic adjuvants to anticancer antibodies. Science 2013; 341: 88-91. PMID: 23722425 DOI: 10.1126/science.1238856. ↩
- Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell 2015; 27: 462-472. PMID: 25858805 DOI: 10.1016/j.ccell.2015.02.015. ↩
- Russ A, Hua AB, Monfort WR, Rahman B, Riaz IB, Khalid MU et al. Blocking “don’t eat me” signal of CD47-SIRPalpha in hematological malignancies, an in-depth review. Blood Rev 2018; 32: 480-489. PMID: 29709247 DOI: 10.1016/j.blre.2018.04.005. ↩
- Allouch A, Voisin L, Zhang Y, Raza SQ, Lecluse Y, Calvo J et al. CDKN1A is a target for phagocytosis-mediated cellular immunotherapy in acute leukemia. Nat Commun 2022; 13: 6739. PMID: 36347876 DOI: 10.1038/s41467-022-34548-3. ↩
- Duluc D, Corvaisier M, Blanchard S, Catala L, Descamps P, Gamelin E et al. Interferon-gamma reverses the immunosuppressive and protumoral properties and prevents the generation of human tumor-associated macrophages. Int J Cancer 2009; 125: 367-373. PMID: 19378341 DOI: 10.1002/ijc.24401. ↩
- Behmoaras, J. & Gil, J. Similarities and interplay between senescent cells and macrophages. J Cell Biol 2021; 220: e202010162. PMID: 33355620 DOI: 10.1083/jcb.202010162. ↩
- Tonnessen-Murray CA, Frey WD, Rao SG, Shahbandi A, Ungerleider A, Olayiwola JO et al. Chemotherapy-induced senescent cancer cells engulf other cells to enhance their survival. J Cell Biol 2019; 218: 3827-3844. PMID: 31530580 DOI: 10.1083/jcb.201904051. ↩
- Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer 2009; 9: 400-414. PMID: 19440234 DOI: 10.1038/nrc2657. ↩
- Kramer JL, Baltathakis I, Alcantara OS, Boldt DH. Differentiation of functional dendritic cells and macrophages from human peripheral blood monocyte precursors is dependent on expression of p21 (WAF1/CIP1) and requires iron. Br J Haematol 2002; 117: 727-734. PMID: 12028050 DOI: 10.1046/j.1365-2141.2002.03498.x. ↩
- Comalada M, Xaus J, Sanchez E, Valledor AF, Celada A. Macrophage colony-stimulating factor-, granulocyte-macrophage colony-stimulating factor-, or IL-3-dependent survival of macrophages, but not proliferation, requires the expression of p21(Waf1) through the phosphatidylinositol 3-kinase/Akt pathway. Eur J Immunol 2004; 34: 2257-2267. PMID: 15259023 DOI: 10.1002/eji.200425110. ↩
- Blum S, Martins F, Lubbert M. Immunotherapy in adult acute leukemia. Leuk Res 2017; 60: 63-73. PMID: 28756350 DOI: 10.1016/j.leukres.2017.06.011. ↩