TREM2 acts as a receptor for IL-34 to suppress acute myeloid leukemia in mice
TREM2 acts as a receptor for IL-34 to suppress acute myeloid leukemia in mice
Author: Marta Irigoyen is a postdoctoral researcher at CIC bioGUNE
Acute myeloid leukemia (AML) is a stem cell disease caused by the massive mobilization and differentiation arrest in primordial granulocytes. AML is characterized by a low survival rate and high recurrence 1. Although differentiation-induction therapy represents an innovative treatment strategy for AML 2, many problems still exist, including irreversible resistance, toxic effects, and limitations of the available targeted inhibitors 3. Thus, there is an urgent need for the development of safe and effective therapeutic strategies to induce AML cell differentiation. Triggering receptor expressed on myeloid cells 2 (TREM2) is a cell surface receptor that functions as a major pathology-induced immune signaling 4. Indeed, TREM2 becomes central in sensing signals or cytokines released by the injured tissues, triggering myeloid immune reactions to restrict its spread 5. However, the role of TREM2 in leukemic malignancies, including in AML, has not been reported to date. Interestingly, in the present work, Xie X. and coworkers identified TREM2 as a novel receptor for interleukin-34 (IL-34) and highlighted IL-34 as a promising differentiation therapy for myeloid malignancies 6.
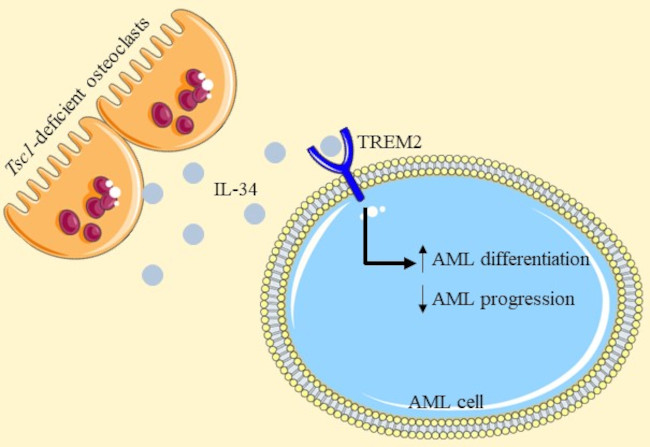
Bone marrow (BM) niches are local microenvironments partly created by accessory cell types, including skeletal cells, which support leukocyte mobilization and differentiation 7. Bone-forming osteoblastic and bone-resorbing osteoclastic lineages are key niche components in the bone, and blockage or ablation of either osteoblast or osteoclast function could impair hematopoiesis 8. In a previous work, the authors found that TSC1 deletion in osteoclast reveal an essential role of TSC1 signaling in the regulation of bone resorption 9. Having this into account, they analyzed the effect of this ablation in AML finding that the overall survival rate was improved and AML cell invasion was reduced in mice where osteoclast-Tsc1 expression was inhibited.
Interestingly, the authors found that IL-34 10 was markedly increased in Tsc1-deficient osteoclasts. To define the anti-AML potential of IL-34, they generated a variety of AML mouse models and they obseved that IL-34 prolonged the animal lifespan. Moreover, this interleukin reduced the expansion of leukemic blasts, and suppressed splenomegaly and invasion into organs of AML mice compared with control-vehicle mice. Next, they carried out an analysis to explore the effects of IL-34 on the gene expression profiles in AML. The obtained results revealed that IL-34 was able to induce a differential expression of multiple genes that are associated with the regulation of leukocyte differentiation. As they expected, IL-34 treatment had a beneficial effect on leukemic cell differentiation, with an increase in the proportion of mature myeloid type in the BM.
Next, they proposed to analyze which receptor was involved in the effects generated by the IL-34 and performed a screen for IL-34 receptors using an AML cell line. Surprisingly, the deletion of CSF1R, PTPRZ1, or SDC (the known IL-34 receptors), did not prevented the inhibitory effect of IL-34 on an AML cell line growth. Therefore, they screened for alternative IL-34 receptors and TREM2 was unexpectedly included with a high score. After performing different approaches, they confirmed that IL-34 was recognized by leukemic cells via TREM2.
To demonstrate the functional involvement of TREM2 in leukemic cell differentiation and AML progression, they established TREM2 knockdown (silencing) in AML primary blasts and two different AML cell lines. Notably, the influence of IL-34 on cell proliferation and colony formation, the biochemical characteristics of cell differentiation, and morphological changes were absent in TREM2-null cells. IL-34 administration also lost its beneficial effect in mice that had received grafts of TREM2-null cells. Indeed, they observed that these animals exhibited higher mortality and accelerated organ invasion compared with placebo therapeutic controls. In addition, mature myeloid phenotypes were not detected in the context of TREM2 deficiency.
Then, they performed specific analysis to obtain information about the signaling cascades of the IL-34-TREM2 interaction in AML cells. The obtained results showed that mitogen-activated protein kinase (MAPK) signaling pathways were mainly enriched after IL-34 stimulation and the degree of MAPK activation was reportedly related to AML malignancy. Besides, it has been previously shown that ERK1/2 signaling, a key component of MAPK family, is a master regulator of cell proliferation and differentiation 11. Thus, they confirmed that IL-34 rapidly dephosphorylated ERK1/2 pathway (inhibited its signaling) in various AML cell lines and AML blasts, whereas TREM2 deletion eliminated the inhibition of ERK1/2 by IL-34.
Finally, they investigated the clinical relevance of IL-34 and TREM2 signaling in patients with AML. Different analysis revealed a decreased of TREM2 levels in the BM of patients with AML respect to the BM of healthy individuals. Besides, patients with AML with high TREM2 levels showed prolonged survival, compared with patients with AML and low TREM2 levels, suggesting a clinical correlation between TREM2 expression and AML prognosis. Respect to IL-34 expression, comparing AML cases with different clinical characteristics, higher IL-34 levels were found in samples from patients in the more differentiated subgroups than in samples from thin immature subgroups. They also observed a positive correlation between the expression of TREM2 and IL-34 in samples from patients with AML, emphasizing that TREM2 and IL-34 both correlated with AML occurrence, thus concluding that they are important prognostic factors for AML.
In summary, the authors found that the development of AML xenotransplants was suppressed in mice with osteoclasts Tsc1 deletion. Tsc1-deficient osteoclasts released a high level of IL-34, which efficiently induced AML cell differentiation and prevented AML progression in various preclinical models. Interestingly, IL-34 inhibited AML independent of its known receptors but bound directly to TREM2. Besides, TREM2-deficient AML cells and normal myeloid cells were resistant to IL-34 treatment and TREM2 was downregulated in patients with AML and associated with a poor prognosis. Thus, in this study they identified TREM2 as a novel receptor for IL-34, indicating a promising strategy for overcoming AML differentiation blockade in patients with AML.
References
- Fröhling S, Scholl C, Gilliland DG, Levine RL. Genetics of myeloid malignancies: pathogenetic and clinical implications. J Clin Oncol. 2005; 23: 6285-95. PMID: 16155011 DOI: 10.1200/JCO.2005.05.010. ↩
- Tenen DG. Disruption of differentiation in human cancer: AML shows the way. Nat Rev Cancer. 2003; 3: 89-101. PMID: 12563308 DOI: 10.1038/nrc989. ↩
- Takahashi S.Current Understandings of Myeloid Differentiation Inducers in Leukemia Therapy. Acta Haematol. 2021; 144: 380-388. PMID: 33221808 DOI: 10.1159/000510980. ↩
- Kober DL, Brett TJ. TREM2-Ligand Interactions in Health and Disease. J Mol Biol. 2017;429: 1607-1629. PMID: 28432014 DOI: 10.1016/j.jmb.2017.04.004. ↩
- Deczkowska A, Weiner A, Amit I. The Physiology, Pathology, and Potential Therapeutic Applications of the TREM2 Signaling Pathway. Cell. 2020; 181: 1207-1217. PMID: 32531244 DOI: 10.1016/j.cell.2020.05.003. ↩
- Xiaoling XieWuju ZhangMin XiaoTiantian WeiYingqi QiuJingyang QiuHao WangZeyou QiuSheng ZhangYating PanLinlin MaoYuhua LiBin GuoWanwen YangYuxing HuShujie HuYan GongJun YangGuozhi XiaoYue ZhangXiaochun Bai; TREM2 acts as a receptor for IL-34 to suppress acute myeloid leukemia in mice. Blood 2023; 141 (26): 3184–3198. doi: https://doi.org/10.1182/blood.2022018619 ↩
- Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014; 505: 327-34. PMID: 24429631 DOI: 10.1038/nature12984. ↩
- Charles JF, Aliprantis AO. Osteoclasts: more than ‘bone eaters’. Trends Mol Med. 2014; 20: 449-59. PMID: 25008556 DOI: 10.1016/j.molmed.2014.06.001. ↩
- Xu S, Zhang Y, Wang J, Li K, Tan K, Liang K, Shen J, Cai D et al. TSC1 regulates osteoclast podosome organization and bone resorption through mTORC1 and Rac1/Cdc42. Cell Death Differ. 2018; 25: 1549-1566. PMID: 29358671 DOI: 10.1038/s41418-017-0049-4. ↩
- Greter M, Lelios I, Pelczar P, Hoeffel G, Price J, Leboeuf M, Kündig TM, Frei K et al. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity. 2012; 37: 1050-1060. PMID: 23177320 DOI: 10.1016/j.immuni.2012.11.001. ↩
- Lavoie H, Gagnon J, Therrien M. ERK signalling: a master regulator of cell behaviour, life and fate. Nat Rev Mol Cell Biol. 2020; 21: 607-632. PMID: 32576977 DOI: 10.1038/s41580-020-0255-7. ↩