From within our brain and kidneys, we could have the protein of youth.
From within our brain and kidneys, we could have the protein of youth.
Author: José R. Pineda got his Ph.D. from University of Barcelona in 2006. Since 2007 he has worked for Institut Curie and The French Alternative Energies and Atomic Energy Commission. Currently he is a researcher of the UPV/EHU. He investigates the role of stem cells in physiologic and pathologic conditions.
Aging is inevitable, but not all forms of aging are the same. While some people reach old age in good physical and mental health, others suffer a progressive decline that affects their quality of life. What if there was a way to tip the balance toward healthier aging? A group of researchers from several scientific institutions has explored this possibility through a protein called Klotho, and the results of their study in mice are so promising that they seem like science fiction.
The Klotho protein is synthesized primarily in the kidneys (in tubules called distal convoluted tubules, where sodium and calcium are reabsorbed and potassium and hydrogen ions are secreted in the urine). However, in brain regions such as the choroid plexus (where cerebrospinal fluid is generated) and in neurons of the hypothalamus also has been reported to be found 1. To a lesser extent, it is also expressed in the parathyroid glands, liver, pancreas, and heart. The transmembrane form of Klotho acts as a co-receptor for FGF23 (Fibroblast Growth Factor 23), regulating phosphorus and vitamin D metabolism. The soluble form s-Klotho (s-KL), is derived by proteolytic cleavage or alternative splicing, and has been found to be released circulates throughout the body promoting antioxidant and neuroprotective effects. Because of the protective effects against aging, to assess its potential, Joan Roig-Soriano and collaborators designed a treatment based on gene therapy 2. They used a modified, harmless virus as a vehicle to introduce the s-KL gene into the bodies of healthy mice. This virus, known as AAV9, was administered both intravenously and directly into the brain, ensuring that the protein reached every corner of the body, including the central nervous system.
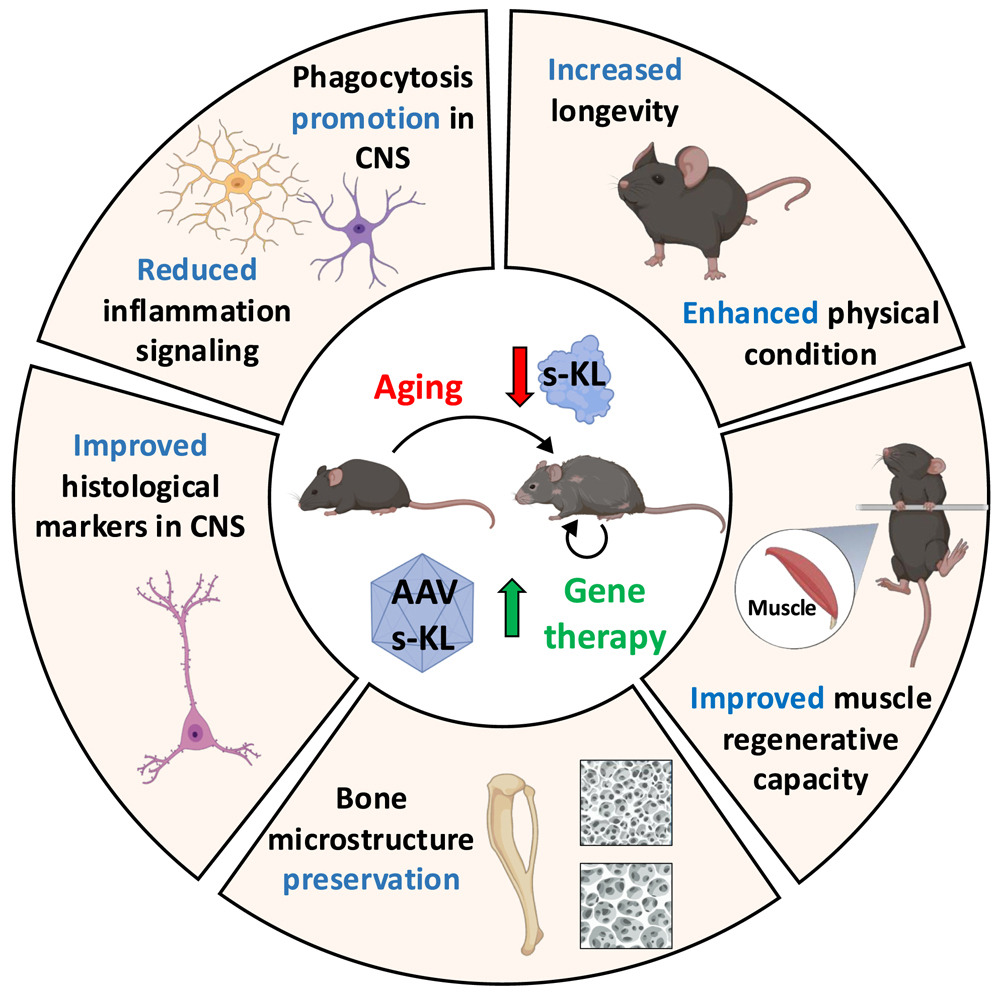
The treatment was administered at two different times in the mice’s lives: at six months, when they were already young adults, and at twelve months, a stage equivalent to middle age in humans. A control group that did not receive the gene was also maintained, allowing the effects of the treatment to be compared with the natural course of aging. From then on, long-term follow-up began. One of the first results that caught attention was the increase in longevity. The treated mice lived, on average, 20% longer than the untreated ones at twelve months. However, the most interesting thing was not only that they lived longer, but that they did so in much better health.
To assess this, the researchers performed a battery of physical tests. In the rotarod test (this is a behavioural test to measure both balance and motor coordination), the treated mice showed superior performance. They also excelled in the horizontal bar test, which assesses strength and endurance while hanging from a bar, and in the grip strength test, which measures muscle power. These results indicated that the animals not only lived longer, but also better maintained their physical capacity with age. Next, they wanted to characterise at histological level what was happening inside the body. Therefore, they analyzed the mice’s muscles under a microscope. They discovered that those treated with s-KL had larger muscle fibers and less fibrous tissue, a clear sign of less muscular aging. They also conducted xenotransplant experiment to determine if the effects were intrinsic or extrinsic (due to the microenvironmens), a sort of approximation that has previously done in other pathologies 3: they transplanted muscles from old, treated mice into young mice. What they observed was that these transplanted muscles regenerated better, with more active stem cells in the process of becoming new muscle fibers. This experiment suggested that the s-KL overexpression treatment not only preserved the muscle but also improved its regenerative capacity.
The study also focused on bones, another part of the body that strongly suffers with age. Using high-resolution scanners, the researchers analyzed the bone structure of the mice. They found that those treated with s-KL, especially the females treated at six months, had denser bones, with greater trabecular volume and fewer empty spaces. These changes are important because they indicate greater bone strength and potential protection against osteoporosis. At the molecular level, an increase in the expression of genes related to bone formation, such as type I collagen and osteocalcin, was detected, reinforcing the idea that s-KL promotes bone health.
Because Klotho is also synthetized in the choroid plexus of the brain, the authors also decided to have a look at the brain. Hippocampus is a key region for memory and learning where neurogenesis occurs at its dentate gyrus (a specific zone containing the neurogenic niche). They found more young neurons and astrocytes in the treated mice, as well as greater activity of microglia (brain immune cells). Microglia is essential for maintaining a clean and functional brain environment, and their activation in this context was not associated with inflammation (one of the most detrimental effects that occur in neurodegenerative diseases), but rather with an improvement in the brain’s ability to clean and regenerate.
After observing all the beneficial effects on muscles, bones and especially brain they wanted to better understand the cellular intrinsic changes. To do this, they analyzed which genes were active in the hippocampus. They compared young mice, untreated old mice, and old mice treated with s-KL. They found that the treated mice had a genetic profile more similar to that of young mice, with fewer genes altered by aging. Furthermore, pathways related to beneficial immune responses and the removal of cellular waste were activated, suggesting a healthier brain environment.
In conclusion, this work paves the way for understanding the benefits of increasing soluble Klotho (s-KL) levels in the body, demonstrating their therapeutic potential across multiple organs and functions, particularly for the middle-aged and elderly population.
References
- Akihiro Imura, Akiko Iwano, Osamu Tohyama, Yoshihito Tsuji, Kazuhiko Nozaki, Nobuo Hashimoto, Toshihiko Fujimori, Yo-Ichi Nabeshima (2004) Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. doi: 10.1016/j.febslet.2004.03.090. ↩
- Joan Roig-Soriano, Ángel Edo, Sergi Verdés, Carlos Martín-Alonso, Cristina Sánchez-de-Diego, Laura Rodriguez-Estevez, Antonio L Serrano, Carmela R Abraham, Assumpció Bosch, Francesc Ventura, Bryen A Jordan, Pura Muñoz-Cánoves, Miguel Chillón (2025) Long-term effects of s-KL treatment in wild-type mice: Enhancing longevity, physical well-being, and neurological resilience. Mol Ther. doi: 10.1016/j.ymthe.2025.02.030. ↩
- Pineda JR, Daynac M, Chicheportiche A, Cebrian-Silla A, Sii Felice K, Garcia-Verdugo JM, Boussin FD, Mouthon MA. (2013) Vascular-derived TGF-β increases in the stem cell niche and perturbs neurogenesis during aging and following irradiation in the adult mouse brain. EMBO Mol Med. doi: 10.1002/emmm.201202197. ↩