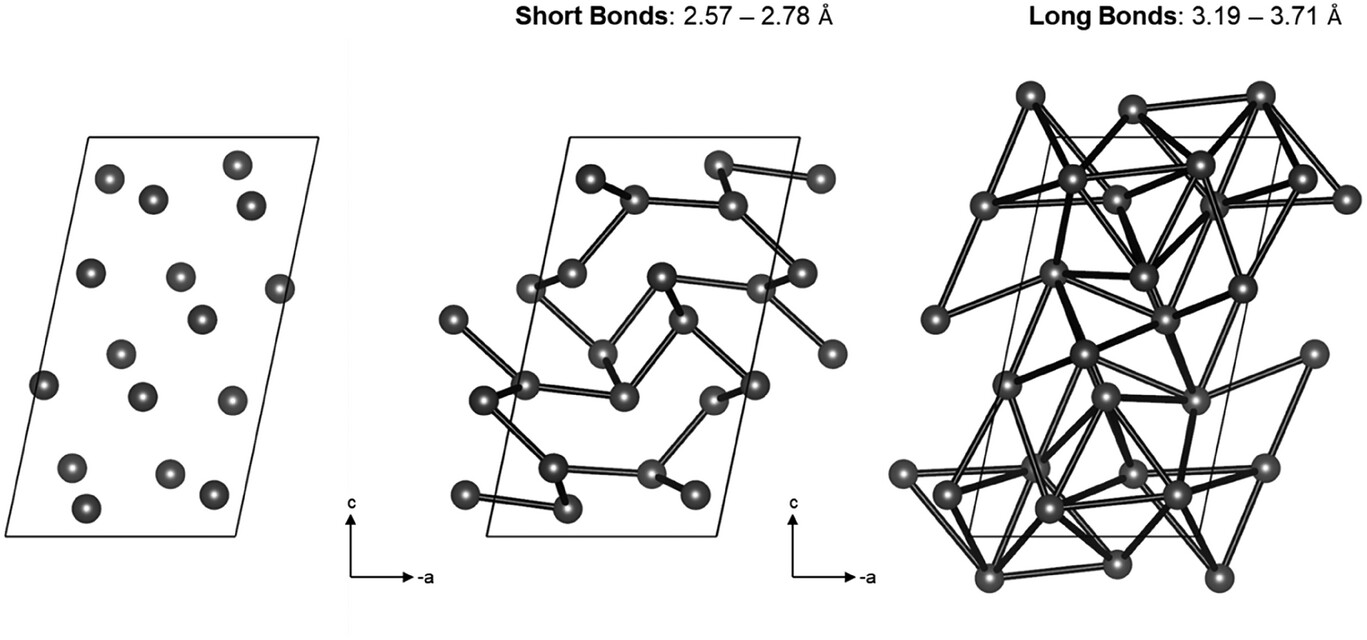
Covalent bonds found in alpha plutonium
Covalent bonds found in alpha plutonium
Plutonium has captured the attention of scientists since its discovery in the early 1940s. This enigmatic element has an important role to play in emerging energy technologies like nuclear batteries and reactors, but it also has complicated electronic behavior that causes some intriguing effects. Its electron structure contributes to unconventional entropic properties at low temperatures, multiple phase transitions before melting, and complex bonding patterns. To add further complexity, plutonium is also found in several different allotropes—forms of the same element with different atomic arrangements. Plutonium’s alpha phase (α-Pu), for example, has a particularly complicated atomic structure.
Although scientists are eager to explore these intriguing properties, studying plutonium remains notoriously difficult. Its strong electronic correlations, complex quantum effects like spin-orbit coupling, and changes due to radioactive decay make both experimental research and computer simulations challenging. Understanding the complex bonding of this phase seemed like a worthwhile challenge for a newly formed collaboration of scientists to tackle.
Motivated by past theoretical work, a research team led by the U.S. Department of Energy’s (DOE) Los Alamos National Laboratory combined advanced computer simulations and high-precision X-ray measurements to more fully understand how atoms bond in α-Pu. This led to the first ever plutonium experiments at the National Synchrotron Light Source II (NSLS-II), a DOE Office of Science user facility at DOE’s Brookhaven National Laboratory.
The team worked closely with Milinda Abeykoon, lead beamline scientist at the Pair Distribution Function (PDF) beamline at NSLS-II. Their research found 1 a mix of bonding types, including evidence of covalent bonding, where atoms share electrons, which helps explain some of α-Pu’s larger-scale mechanical properties. Their results were recently published in Advanced Functional Materials.
Updated techniques reveal structural complexity in α-Pu
Unlike the regular, highly symmetrical crystal structure of plutonium’s delta phase (δ-Pu), which is more commonly studied, the α-phase has a lot more structural complexity. Its atomic arrangement is highly distorted and exhibits a wide range of atomic bonding distances. Early theories suggested that different strengths of chemical bonds might exist in α-Pu, while largely ruling out covalent bonding. But until now, no one has directly studied those bonds experimentally.
“In the field of plutonium, researchers are often interested in its mechanical properties for nuclear technology applications,” said W. Adam Phelan, a nuclear materials scientist at Los Alamos and co-lead author of this work. “You rarely get this bottom-up, atomistic understanding in plutonium science.”
Again, he noted other results suggested there was little to no covalent bonding, but those results were at odds with the mechanical properties that we understand about this allotrope. “These insights explain why α-Pu has certain macroscale properties.”
To investigate how atoms in α-Pu bond, the team used a technique called pair distribution function (PDF) analysis, which can reveal how atoms move together in a structure—an important behavior in complex or disordered materials. PDF measurements provide valuable information about the local atomic structure.
They combined this experimental study with density functional theory (DFT) calculations to validate their atomistic models. DFT helps scientists study the behavior of electrons at the atomic scale by using a simplified approach that focuses on electron density rather than tracking each electron individually. This enables researchers to model complex materials and chemical systems with practical accuracy and computational efficiency.
“α-Pu is a particularly tricky system to study. Its structure is complex, which generates a lot of information,” said Alexander Muñoz, a computational physicist at Los Alamos and co-lead author of this work. “That can make large-scale trends difficult to discern. We really pushed DFT to its limits for this work.”
Before employing any of these novel experimental techniques, a lot of planning and protocol had to be implemented at NSLS-II. On top of the material challenges of studying plutonium, there are also some practical ones. An experiment like this takes months of preparation and the expertise of several specialized teams.
Plutonium, X-rays, and strict safety protocols
Plutonium is a rare and highly regulated material that is difficult to obtain. It’s also toxic, especially as a powder, and radioactive, demanding stringent safety measures. To ensure the experiment was conducted safely and efficiently, experts from the Lab’s Radiological Control Division, NSLS-II’s management team, and the Environment, Safety, Health, and Quality program provided critical oversight and support.
To start, there were strict limits on how much of the material could be brought on site to study, so the team was only able to work with a few precious milligrams. It’s not just the amount of plutonium in the experiment that’s monitored either. Brookhaven accounts for all special nuclear materials, which are regulated lab wide.
During preparation, those small samples were sealed in a custom-built triple containment system designed to ensure both radiological safety and X-ray transparency. At the PDF beamline, the samples were mounted on translation stages to take measurements behind a lead-shielded hutch that was locked down during the entirety of the experiment. Panoramic cameras monitoring inside the hutch provided the team with real-time observation.
Once the experiment was ready to run, PDF’s flexible setup and high energy range helped the team reach the answers they sought out. Many recent updates and planned upgrades to the PDF beamline have made it the ideal tool for Phelan and his team to study this formidable material. They were able to leverage the beamline’s high energy capabilities to penetrate thick samples, and in future experiments, they can switch to another energy in minutes. For example, the beamline could accommodate the lower energy needed to perform small angle X-ray scattering (SAXS) on a thinner plutonium sample.
A Peierls distortion
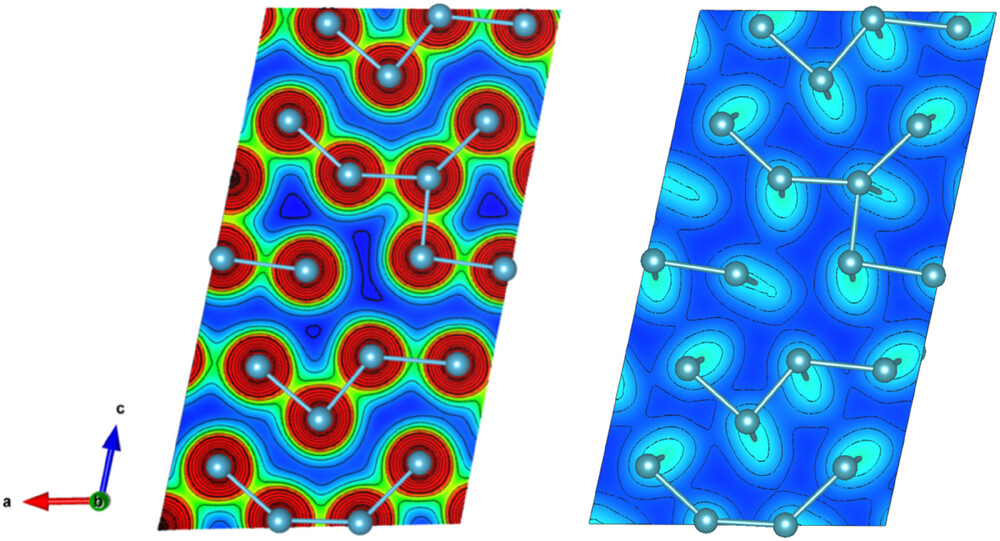
The PDF analysis was combined with a computer-based modeling method called reverse monte carlo. These simulations helped the team identify patterns in the way atoms move together. They found that the atoms in α-Pu move in tightly linked groups, hinting at a significant amount of covalent bonding.
“The model captured the long-range structural order of the first dataset remarkably well,” said Abeykoon. “But the short-range atomic correlations revealed clear deviations from the expected pattern. That was exactly what we were expecting though.”
Scientists used DFT calculations to further analyze the charge distribution and bonding structure, confirming their experimental observations. Their analyses revealed that α-Pu hosts a mix of bonding types: short bonds exhibit directional, covalent-like character, while longer bonds behave more metallically. This mixed bonding landscape aligns with the theory that α-Pu’s structure is shaped by a Peierls distortion, when a material slightly changes the positions of its atoms to lower its overall energy. The presence of covalent bonding helps explain why α-Pu has been observed to behave more like a brittle solid than a malleable metal.
References
- , , , , , , , , , , Experimental and Theoretical Confirmation of Covalent Bonding in α-Pu. Adv. Funct. Mater. doi:10.1002/adfm.202501798 ↩