Strong donor-acceptor coupling does not require covalent bonding
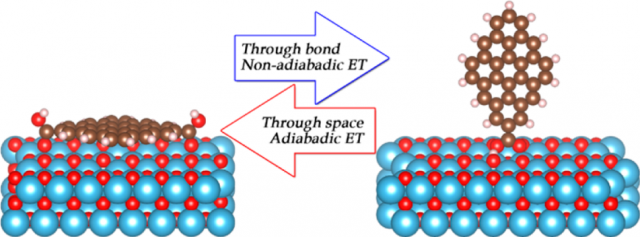
Interfacial electron transfer constitutes the key step in the conversion of solar energy into electricity and fuels. Required for fast and efficient charge separation, strong donor−acceptor interaction is typically achieved through covalent chemical bonding…or not.
Experiences with donor−acceptor molecular diads and triads, conjugated polymers, and DNA, leads to the expectation that a covalent bonding is a must. But calculations Run Long, David Casanova,Wei-Hai Fang, and Oleg V. Prezhdo (2017) Donor−Acceptor Interaction Determines the Mechanism of Photoinduced Electron Injection from Graphene Quantum Dots into TiO2: π-Stacking Supersedes Covalent Bonding JACS doi: 10.1021/jacs.6b09598[/footnoe] by a team of researchers that includes UPV/EHU’s and DIPC’S David Casanova contradicts the idea that strong donor−acceptor coupling requires covalent bonding.
We all know what graphene is, to wit, a two-dimensional form of carbon, composed of a planar hexagonal lattice of carbon atoms connected by both σ-bonds and π-bonds formed, with many unique properties, such as very high thermal and electronic conductivities, and large surface area per unit of mass. We also know that graphene attracts intense attention for a diverse range of applications, including photocatalysis and photovoltaics. But we may not be that familiar with graphene quantum dots.
Graphene quantum dots (GQDs) is a big name for what are simply small pieces of graphene. But being small is important. In addition to the unique properties inherited from graphene, such as high surface area and single atom thickness, GQDs exhibit size behavior. In particular, graphene is a metal, while GQDs have a size-dependent energy gap, which can be tuned to absorb solar photons of any wavelength, from near infrared, to visible, to ultraviolet.
The presence of the energy gap itself, which ensures long-lived excited states, and the gap tunability are strongly desirable in solar energy applications. We can use GQDs, for example, to fabricate solar cells directly, or employ them as photosensitizers interfaced with metal oxides like TiO2 to produce visible-light photocatalytic and photovoltaic devices.
Actually, experiments show that the electron injection from photoexcited GQDs into TiO2 is ultrafast, taking less than 15 femtoseconds. This indicates that GQD/TiO2 composites can yield high photo-to-electron conversion, because the charge separation at the interface is fast and efficient. This finding makes ot extremely interesting to develop a model of the charge separation dynamics at GQD/TiO2 interfaces, in order to develop a thorough understanding of the dynamics and its mechanisms, and to provide chemical guidelines for the development of GQD/TiO2 solar cells.
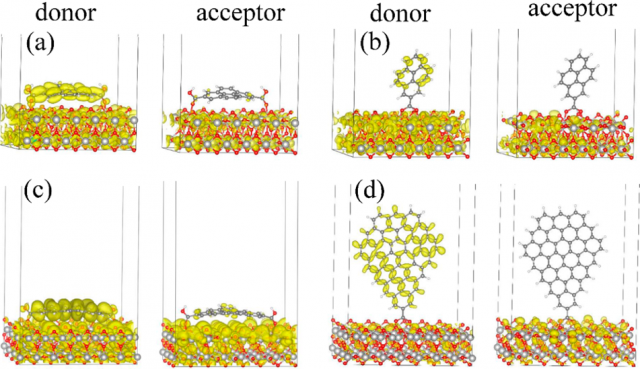
This is exactly what the researchers did. Using real-time time-dependent density functional theory combined with non-adiabatic molecular dynamics, they simulated photoinduced electron transfer into TiO2 from different molecules and GQD containing commonly used carboxylic acid linkers. They investigated how whether they where flat (pyrene, coronene) or vertically (pyrene, GDQ) placed influenced the outcome.
They results show that the carboxilic linker does not provide a sufficiently strong donor−acceptor coupling. This observation is rationalized by the π-electron withdrawing properties of the carboxylic group, and should apply to other commonly used linkers.
But, importantly, the simulations reveal a strong and counterintuitive dependence of the donor−acceptor coupling and electron transfer mechanism on the type of chemical bonding. The researchers show that covalent bonding produces weak donor−acceptor coupling and gives the nonadiabatic electron transfer mechanism.
These conclusions have a broader impact as they should apply to other heterojunctions, including interfaces of bulk semiconductors with large molecular chromophores and a variety of linker moieties, with two-dimensional materials, such as graphene and MoS2, as well as to multicomponent nanoscale systems in general.
These principles would allow designers to apply chemical tools in order to tune the properties of hybrid nanoscale materials for use in electronic and spintronic devices, solar energy harvesting and utilization, etc.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance.
1 comment
[…] La suposición de que para que exista una buena transferencia electrónica entre un aceptor y un donante en los sistemas que se emplean para transformar energía solar en electricidad tiene que existir un enlace covalente ha resultado ser falsa […]