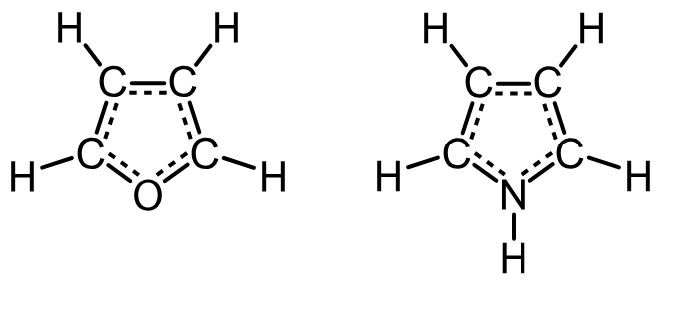
Using light to replace an oxygen atom with a nitrogen atom in a furan molecule
A team of chemists at the Korea Advanced Institute of Science and Technology has succeeded in pulling an oxygen atom from a molecule and replacing it with a nitrogen atom. In their study, published 1 in the journal Science, the group used photocatalysis to edit a furan in their lab.
Ellie Plachinski and Tehshik Yoon with the University of Wisconsin–Madison, have published a Perspective piece 2 in the same journal issue outlining the process and explaining how it could be used to change the way that drugs are made.
Prior research has shown that some complex molecules can be edited using chemical reactions, but they are few and far between. So chemists must synthesize molecules from scratch when they want to change a small part of a molecule, or even just one atom, for testing.
Such work has shown that even minor changes can have a major impact—as Plachinski and Yoon note, changing a single atom in a heterocycle can have a profound impact on the efficacy of a drug. Chemists have been looking for more efficient ways to edit molecules—or more specifically, to remove a single atom and replace it with another.
In this new study, the research team developed a technique they describe as a pencil-and-eraser technique in which one atom is erased and another penciled in.
The researchers were inspired by a paper written by chemists Axel Couture and Alain Lablache-Combier in 1971, in which they used ultraviolet light to convert a furan to a N-propylpyrrole as a way of improving yield. They used ultraviolet light to swap an oxygen atom in a furan with a nitrogen atom.
Such editing, the team notes, is particularly challenging due to issues with delocalization—prior attempts have involved applying high temperatures or radiation. Neither approach has been found to be suitable.
In this new approach, the team used light as a photocatalyst for activating a furan ring. The technique can be used to carry out single electron oxidation on a furan, resulting in radicalization.
The approach, they note, allows for a facile reaction that is susceptible to the addition of an amine. That leads to a cascade of electron and proton transfer between the product and the photocatalyst, resulting in the creation of a ring aldehyde intermediate.
References
- Donghyeon Kim et al. (2024) Photocatalytic furan-to-pyrrole conversion. Science doi:10.1126/science.adq6245 ↩
- Ellie F. Plachinski, Tehshik P. Yoon (2024) Single-atom editing with light. Science doi:10.1126/science.ads2595 ↩