First video of catalysis in action at the atomic level
First video of catalysis in action at the atomic level
An international team of scientists has, for the first time, directly observed 1 catalysis in-action at the atomic level.
In mesmerizing new videos, single atoms move and shake during a chemical reaction that removes hydrogen atoms from an alcohol molecule. By viewing the process in real time, the researchers discovered several short-lived intermediate molecules involved in the reaction as well as a previously hidden reaction pathway.
The observations were made possible by single-molecule atomic-resolution time-resolved electron microscopy (SMART-EM), a powerful instrument that enables researchers to watch individual molecules react in real time.
Observing reactions in this manner helps scientists understand how catalysts work. These new insights could potentially lead to designs for more efficient and sustainable chemical processes.
Live catalytic events
Researchers have long sought to observe live catalytic events at the atomic level. Chemical reactions are like a journey between starting materials and the final product. Along the journey, transient—and sometimes unexpected—molecules form and then abruptly transform into other molecules. Because these so-called “intermediate” molecules are unpredictable and fleeting, they are difficult to detect.
By directly watching the reaction unfold, however, scientists can determine the exact sequence of events to reveal the complete reaction pathway—and view those elusive intermediates. But, until recently, it was impossible to observe these covert dynamics.
While traditional electron microscopes can image atoms, their beams are too strong to image the soft, organic matter used in catalysis. The high-energy electrons easily break down carbon-based structures, destroying them before scientists can gather the data.
To overcome this challenge, the team turned to SMART-EM, a novel technique that can capture images of delicate organic molecules. Unveiled by Eiichi Nakamura and his team at the University of Tokyo in 2018, SMART-EM uses a much lower electron dose, minimizing the amount of energy—and damage—transferred to the sample. By capturing rapid sequences of images, SMART-EM generates videos of dynamic processes, which Nakamura calls “cinematic chemistry.”
From messy to measurable
When applying SMART-EM to catalysis for the first time, the team chose a simple chemical reaction: removing hydrogen atoms from an alcohol molecule. But first they needed to select the right catalyst. About 85% of industrial catalysts are heterogeneous, meaning they are solid materials that react with liquids and gases.
Although heterogeneous catalysts are stable and efficient, they are also messy, with many different surface sites where reactions might occur. To make the catalyst easier to study, the researchers designed a single-site heterogeneous catalyst with a well-defined active site. The single-site catalyst comprised molybdenum oxide particles anchored to a cone-shaped carbon nanotube. Then, the team used SMART-EM to investigate how their catalyst facilitated the conversion of ethanol into hydrogen gas, a clean alternative to fossil fuels.
Unveiling a hidden pathway
Before the study, scientists posited that alcohol went straight to the catalyst, where it became hydrogen gas and aldehyde (a molecule that forms when an alcohol molecule oxidizes). From there, the aldehyde, which is a gas at room temperature, escaped into the air. But watching the process unfold revealed a different story.
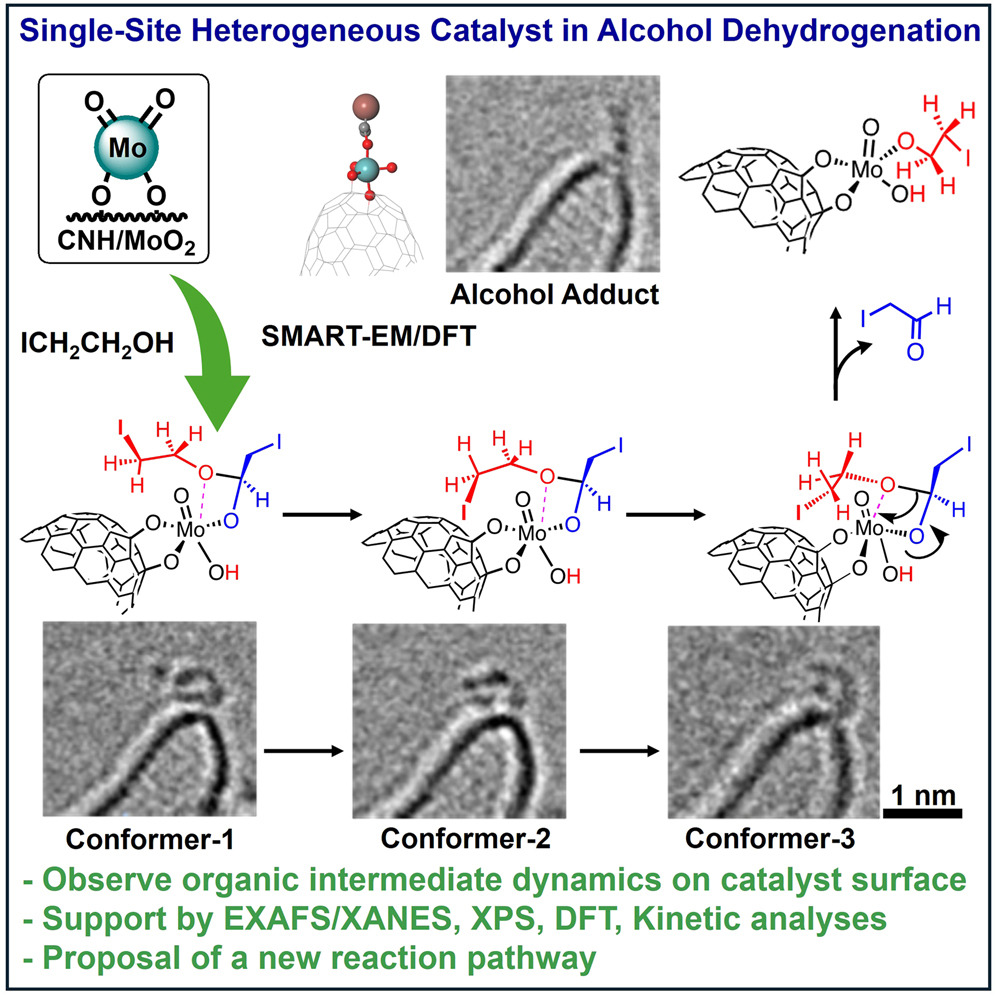
Using SMART-EM, the researchers discovered the aldehyde doesn’t float away, but instead sticks to the catalyst. They also found the aldehydes linked together to form short-chain polymers—a previously unknown step that appeared to drive the overall reaction. In another surprise, the researchers discovered the aldehyde also reacts with alcohol to form hemiacetal, an intermediate molecule that is then converted into other products.
To confirm these findings, the team used various microscopy techniques, X-ray analysis, theoretical models and computer simulations. All matched the SMART-EM data.
“Since 2007, physicists have been able to realize a dream over 200 years old—the ability to see an individual atom,” Nakamura said in a 2019 statement. “But it didn’t end there. Our research group has reached beyond this dream to create videos of molecules to see chemical reactions in unprecedented detail.”
References
- Kratish, Yosi et al. (2025) Atomic-resolution imaging as a mechanistic tool for studying single-site heterogeneous catalysis Chem doi: 10.1016/j.chempr.2025.102541 ↩