A cooler way to see inside the body: Cryogenic cesium iodide for medical imaging
A cooler way to see inside the body: Cryogenic cesium iodide for medical imaging
Positron Emission Tomography, or PET, is one of medicine’s most powerful tools for looking inside the human body, revealing not only anatomy but also how tissues are functioning. In a PET scan, a patient receives a tiny dose of a radioactive tracer that emits positrons, the antimatter counterparts of electrons. When a positron encounters an electron, the two annihilate, releasing a pair of gamma rays that speed away in opposite directions, each carrying an energy of 511 keV. By detecting these gamma rays, the scanner can reconstruct a detailed, three-dimensional image of processes taking place within the body.
Costly LYSO
The most advanced machines today, called Total Body PET scanners, can capture the entire body at once. This increases sensitivity dramatically, allowing scans to be completed more quickly or with far smaller doses of radioactivity. However, this leap in capability comes with an enormous price tag, placing these machines out of reach for many hospitals. A large part of that cost comes from the scintillator material used to detect the gamma rays. Most commercial PET systems rely on a crystal called LYSO, short for lutetium yttrium oxyorthosilicate. LYSO is fast, bright, and dense, making it almost ideal for PET imaging, but it is expensive to produce and requires high manufacturing temperatures. Filling the length of an entire body scanner with LYSO crystals is prohibitively costly.
Can cooling cesium iodide do the trick?
Now, a team of researchers explores 1 an alternative: pure cesium iodide, or CsI, operated at cryogenic temperatures around 100 kelvins—just above the boiling point of liquid nitrogen. At room temperature, CsI has never been a top contender for PET. It produces less light than LYSO, and that light is in the deep ultraviolet range, which many detectors do not pick up efficiently. Cooling the material, however, changes its behaviour dramatically. When the temperature drops to about 100 K, CsI’s light output increases about twentyfold, reaching roughly one hundred photons for every kiloelectronvolt of gamma-ray energy deposited. At the same time, the colour of its light shifts from around 310 nanometers, deep in the ultraviolet, to about 340 nanometres, a range that matches much better with the sensitivity of modern silicon-based light sensors.
Cooling comes with a trade-off. The time it takes for the crystal’s light to fade—its decay time—becomes much longer. At room temperature, CsI emits most of its light in about fifteen nanoseconds, but at 100 K this stretches to roughly eight hundred nanoseconds. In PET imaging, timing is important because scanners record not just the energy of the incoming gamma rays but also the precise moment they arrive. A longer decay time can make it harder to determine that moment accurately. In this case, however, the much brighter light partly offsets the loss in timing precision.
Brighter light vs timing precision
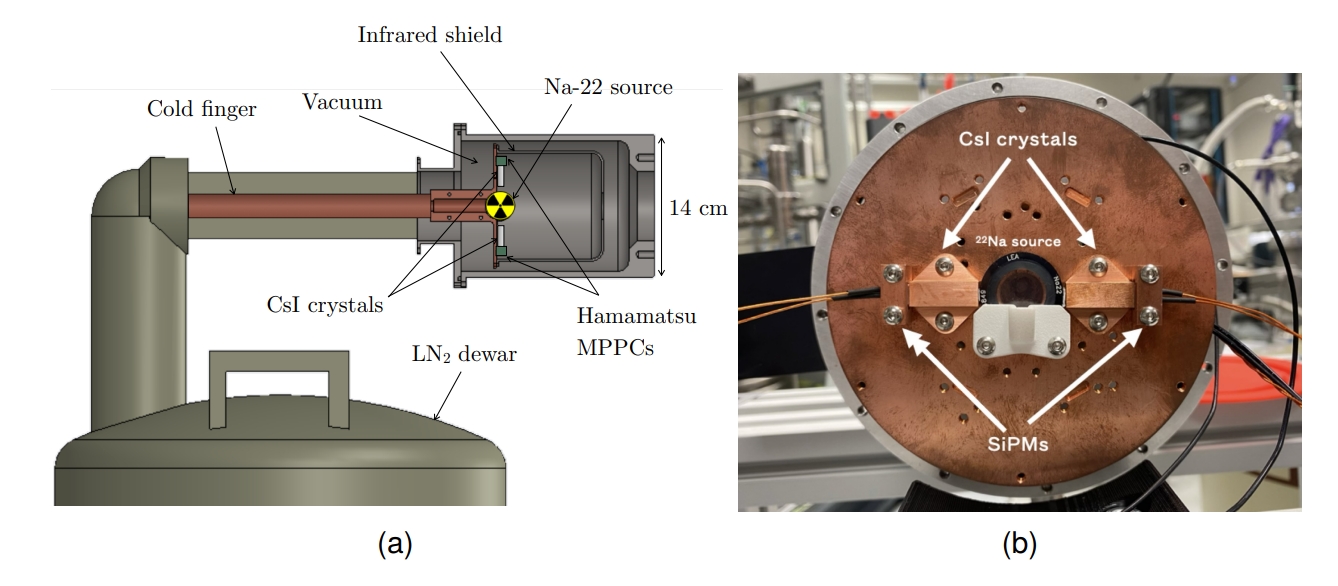
To explore this balance, the researchers tested two pure CsI crystals, each a few millimeters across and two centimeters long. They wrapped the crystals in reflective tape, placed them in a vacuum cryostat, and cooled them using a copper “cold finger” connected to a liquid nitrogen reservoir. They used a sodium-22 source, which produces the same 511 keV gamma rays as a PET tracer, and detected the light using small silicon photomultipliers.
At room temperature, the crystals produced about 4,500 photons per MeV of energy, and the energy resolution for 511 keV gamma rays was around 35 percent—far too poor for PET imaging. At 104 K, the light yield soared to nearly 90,000 photons per MeV. This remarkable increase brought the energy resolution down to about 6.3 percent, better than many commercial PET crystals. The researchers also measured coincidence time resolution, a metric for how precisely the scanner can register the difference in arrival times of two gamma rays from the same annihilation event. At room temperature the value was 1.31 nanoseconds; at 104 K it rose to 1.84 nanoseconds because of the slower light emission, but this was still within acceptable limits for PET use.
Cryogenic cesium iodide could even outperform LYSO
These results are significant because they suggest that cryogenic CsI could rival or even outperform more expensive materials like LYSO in terms of energy resolution, while offering adequate timing for medical imaging. Given that CsI costs several times less than LYSO and is also cheaper to manufacture, the savings could be enormous when building large Total Body PET systems. The expense and complexity of cooling the detectors would be a factor, but the economics could still favor CsI for many institutions.
Looking forward and beyond
Looking forward, the team plans to scale up their experiments, testing larger arrays of sensors and possibly single, large crystals. They also want to explore measuring the depth at which gamma rays interact within the crystal, a feature that could further improve image sharpness. Another avenue is the detection of Cherenkov photons, a very fast type of light emitted by charged particles moving faster than light in the crystal, which could help recover some of the lost timing performance.
While the focus is on PET, the implications go beyond it. Other imaging methods, such as SPECT, could also benefit from cryogenic CsI’s high energy resolution, even though its longer decay time would limit its use in applications requiring extreme timing precision. In short, by making cesium iodide cold enough to sparkle with far greater intensity, this research shows a possible path toward more affordable and accessible high-performance medical imaging.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research paper/s.
References
- S.R. Soleti, A. Castillo, M. del Barrio-Torregrosa, C. Echeverria, M. Seemann, D. Zerzion, J.J. Gómez Cadenas and J.I. Collar (2025) Cryogenic cesium iodide as a potential PET material JINST doi: 10.1088/1748-0221/20/06/P06038 ↩