This acrobatic behavior had been observed for more than a hundred years, yet only in 2024 did scientists finally understand how L. olor manages to whip out and store its neck so deftly. The tiny hunter uses a kind of cellular origami: It folds its external membrane in pleats that it can unfold, deploy and retract at will.
“This particular origami, which we named Lacrygami — humans did not invent it, nature invented it,” says Stanford University bioengineer Manu Prakash.
Like releasing tightly spooled fishing line, the tiny, single-celled hunter Lacrymaria olor can rapidly extend its neck 30 times its body size and just as quickly whip it back into itself.
Anyone who has dabbled in origami knows that it can be frustratingly complicated, yet somehow its intricate folds have arisen naturally many times in living things. In recent years, scientists have taken a closer look at these complex folds of the biological realm, such as in delicate insect wings, a chick’s developing gut or the lightning-fast neck of L. olor.
Some of what they’re finding is inspiring practical applications such as drones and robots, but the nature of origami itself is enough to keep scientists fascinated. Origami exists at a particular boundary, says Harvard University physicist Lakshminarayanan Mahadevan, “where there is just enough balance between constraints and freedom, so that you can do remarkable things.”
Frontiers in space
Japanese people started practicing origami some time around the sixth century, but it wasn’t until about 40 years ago that scientists and engineers began investigating origami in earnest. Early studies focused on usefulness in space: With origami, one could tightly pack solar panel arrays on a rocket for unfolding later on.
Japanese astrophysicist Koryo Miura published what became a standard folding technique for such applications in 1985. Called the Miura-ori, this rigid fold is made of mountain and valley creases; it’s essentially a pattern of closely packed parallelograms. With one pull, you can unfurl an entire folded sheet, such as a map or an array of solar panels, and then just as easily fold it up again. In 1995, the fold was used to efficiently pack solar panel arrays in Japan’s Space Flyer Unit satellite.
The Miura fold, or Miura-ori has been used to compactly fold structures such as solar arrays that can then be unfolded in a single motion.
But long before then, the fold was deployed in nature. In a classic 2005 paper published in Science, Mahadevan and physicist Sergio Rica, currently at Pontificia Universidad Católica de Chile, posited that a Miura-ori-like pattern could naturally occur in leaves or insect wings, due to inherent physical instabilities. Using mathematical models and a drying slab of gelatin, they demonstrated how light compression on a stiff, thin skin that’s supported by a soft, thick substrate can prompt the skin to settle into a Miura-ori like pattern, akin to how compression of the Earth’s crustal plates can lead to mountains and valleys.
More recently, Mahadevan and his team investigated how different parts of a chick gut — the large intestine with wrinkles, for example, the small intestine with zigzag folds — develop their very different creases. It turns out that the layers of gut tissue vary in thickness and stiffness across each portion. As the gut elongates during development, their mechanical properties cause these portions to buckle in different ways, the researchers reported in the Proceedings of the National Academy of Sciences in 2024. Other complex biological folds — like the wrinkles on our brain — are also likely to form during development due to similar physical force considerations.
“This is essentially a very natural consequence of pattern formation in physics,” Mahadevan says.
Insights from insect wings
Scientists are also investigating how insects neatly fold and unfold their wings. Andres Arrieta, a mechanical engineer at Purdue University, and André Studart, a materials science engineer at ETH Zurich, turned to earwigs, which have hindwings tucked away under their forewings. Just before flying, the earwig unfurls the hindwings, and all the tightly packed creases elegantly open as thin, delicate wings stretching out to more than 10 times their folded size. The process takes place without the use of muscles.
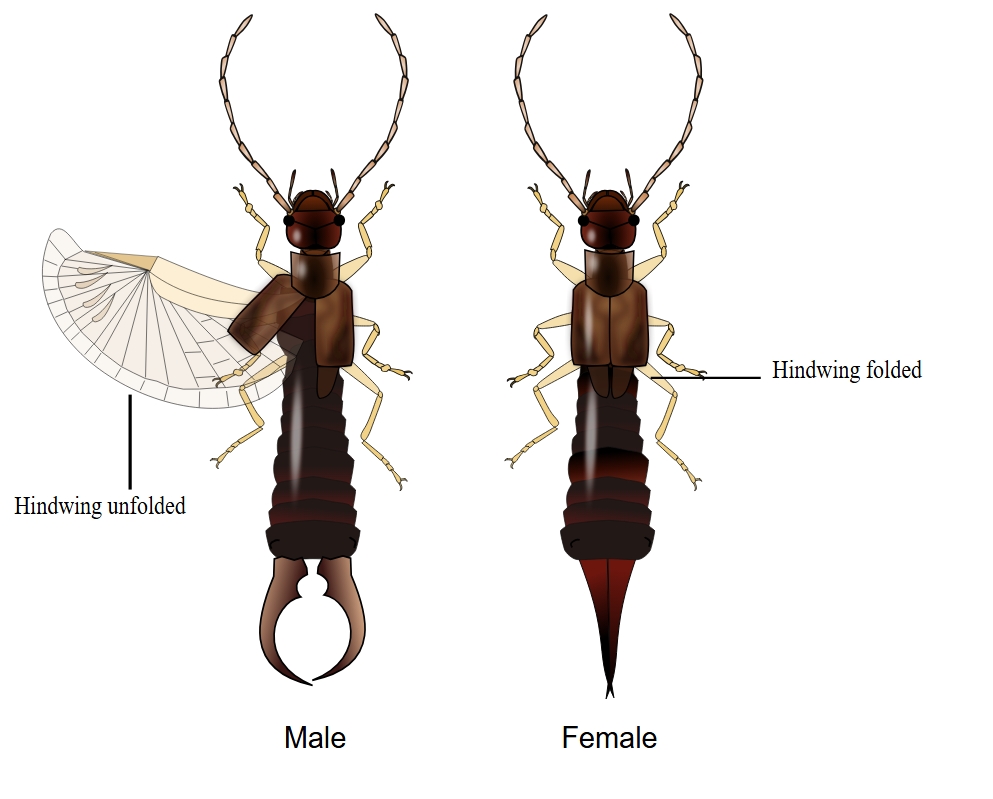
The researchers were drawn to the earwig wing for three reasons: It has a large change in area as it folds or unfolds; it’s what scientists call bistable (it can be at rest in two different states, open and folded); and it doesn’t just have standard straight origami creases — it has curved creases.
Curved creases don’t fold flatly along a straight line, they fold along a curve, like a folded-over shirt collar. They are trickier than a standard flat crease; as you fold along a curve, the crease changes direction ever so slightly at every point. So, at each point, the sheet needs to fold in two directions: radially along the crease, where the two parts of the sheet bend like a hinge and come closer, and tangentially to the crease. Because the crease is curved, every part of it is angled slightly differently.
It turns out that earwigs stretch their wings ever so slightly at the curved crease along the wing’s middle. It manages this stretching with an elastic protein called resilin that can store and release energy like a spring. In the middle of the earwig wing — a spot the researchers call the mid-wing mechanism — the resilin is distributed both symmetrically and asymmetrically. The former helps the wing’s creases extend, like a stretchy spring, and the latter gives the creases the energy to rotate, like a bendy spring. Together, the two types of springs help to lock the wing in position, whether folded or unfolded.
Incorporating the stretchy springs into the folds was key to capturing the behavior of the wing, says Arrieta, who calls the approach “spring origami.”
After modeling how the insect wing folded mathematically, the researchers designed and 3D-printed a membrane that incorporated springs and could fold up on its own.
With some origami applications, the creases have to be folded in the right order to get the final shape. That requires a lot of control, says Arrieta. In contrast, a bistable structure has only two states, open and closed. “It’s just a little bit of effort and boom! the thing deploys.”
Eventually these bistable, foldable structures might be deployed as wings for drones, helping them fold up more compactly.
Lo and be-fold
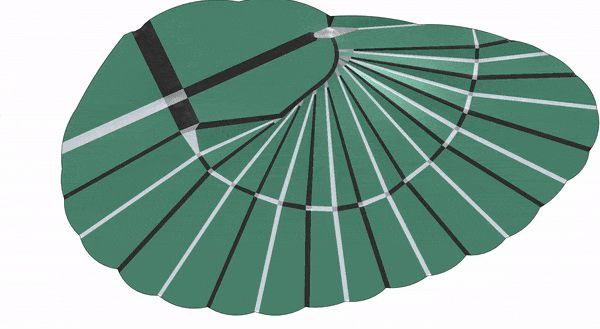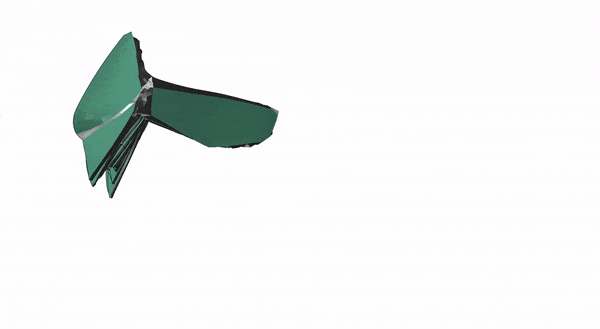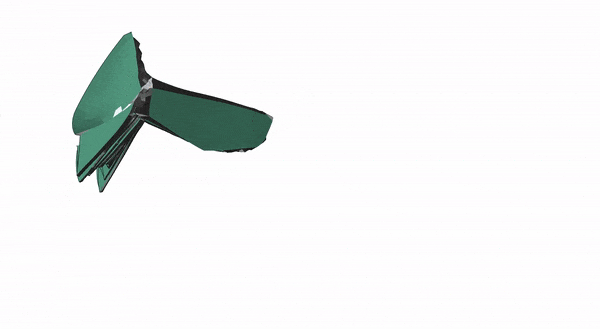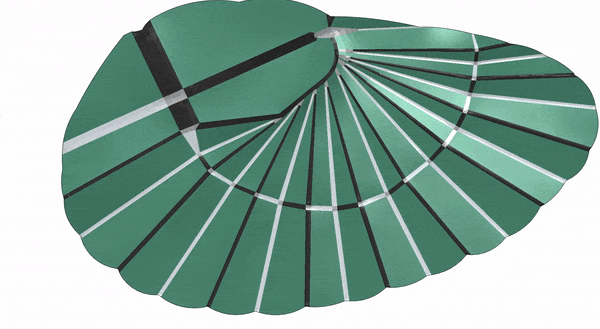
The single-celled hunter L. olor presented a similar puzzle — and a similar solution. The scientists knew that the protist’s body had microtubule proteins that give it a helical structure, the way rods give tents their shape. But could those microtubules help explain its massively extending, then retracting, neck?
After all, the membrane can’t just appear and disappear, says Prakash, so where does it come from and where does it go?
On a trip to Japan, Prakash saw chochin lanterns that have paper stretched over bamboo frames and realized that the membrane of the single-celled creature might similarly stretch out over the bamboo-like microtubules. By testing this paper-based set-up using origami with his kids, he discovered that “there is a very easy way to fold and unfold this architecture.”
Cross-checking with L. olor’s microscopy data confirmed his hunch: The protist’s cell is folded up into pleats using curved creases, and anchored to a scaffold of helical microtubules. Opening and closing of these pleats drives the extraordinary extending neck.
Folding and storing the membrane and microtubules in this curved, helical fashion allows the cell to keep a lot of its gelatinous cytoplasm in a ready-to-release configuration. But L. olor doesn’t just release all that cytoplasm at once, says study coauthor Eliott Flaum, Prakash’s former graduate student who is now a biophysicist at the European Molecular Biology Laboratory in Heidelberg, Germany. “It can control the neck length,” she says. “And that is only possible if it had really fine control over the material being stored.”
It exerts this fine control with the help of what are called singularities — points or kinks along the curved creases where the membrane sharply goes from being folded to being unfolded. Similar to resilin and the mid-wing mechanism in the earwigs, these points concentrate a lot of the bending energy when the membrane is all folded up. And by controlling how these points move, L. olor is able to rapidly unfurl its pleats and just as easily fold them back up again.
As the little hunter either deploys or reels its neck back in, the singularities move along with the neck — ensuring that all the creases open and fold back in sequentially — in the same way every time. Thus L. olor perfectly folds and unfolds its origami without fail — like the pleats of an accordion that open and tuck themselves back in.
“Mathematically, it does not allow any other folds,” says Prakash, “which is why it’s so robust — the cell folds and unfolds tens of thousands of times and does not make a mistake.”
This contrasts with some forms of rigid origami and their straight creases, which can be folded in various ways. Thus, when working with a complex pattern, “it’s actually much more complicated to figure out how a fold pattern is going to fold than you might have guessed,” says physicist Christian Santangelo of Syracuse University. “For any given fold pattern, there’s potentially a very large number of ways of folding it,” says Santangelo, who wrote a review on self-folding origami in the Annual Review of Condensed Matter Physics. “So, if there’s any mistakes in the folding process or design, you might not get what you want.”
That’s partly why origami remains a never-ending source of curiosity for scientists. “Origami is not something that you just play with,” Prakash says. “You can be done with it — it’s never done with you.”