Is science ready to supply serviceable cardiomyocytes?
Is science ready to supply serviceable cardiomyocytes?

The stem cell research field shocked the scientific community back in 2006 thanks to Shinya Yamanaka, who found a way to obtain pluripotent stem cells from adult somatic cells 1. The recipe consisted of just four genes encoding for transcription factors allowing him to reprogram mouse fibroblasts into induced pluripotent stem cells (iPSCs). The “Yamanaka factors” included Oct4, Sox2, cMyc and Klf4, just enough to earn him a Nobel Prize six years later (shared with Sir John Gurdon). Ten years later, the reprogramming process has been improved and refined but more important, we are now able to get cells from any human tissue we can imagine. Still, there seems to be a particular type where progress has not been as quick as scientists would have liked. These are the cardiac muscle cells.
Although the heart is a remarkable pump, able to work continuously for even longer than 100 years and pumping a total of 450 million liters of blood, is not a perfect one. Heart failure is a major burden to our society with millions of new individuals being affected every year. Among those, up to 50% may suddenly die, likely as a consequence of lethal cardiac arrhythmias 2. Hence, it seems convenient the effort scientists make trying to obtain and characterize iPSCs-derived cardiomyocytes (iPSCs-CM).
There are three areas that may benefit from iPSCs-CM 3. The first is screening and drug discovery, mainly because it relies on in vitro and animal models which do not accurately recapitulate the events triggering heart disease. iPSC-CM would therefore substantially improve preclinical studies output and would save time and money when developing new medicaments. The second area is cell therapy, where cells from the patient himself could be used to generate iPSCs-CM which could then be used to treat a broad range of heart pathologies. Finally, we can obtain iPSCs-CM from patients with an inherited cardiac disease to better understand the steps or processes leading to the abnormal physiology. This would be the area of disease modelling. Unfortunately, we still lack knowledge about molecular details related to key processes happening in these cells, including Ca2+-handling.
The first studies obtaining CM derived from mouse were published in 2008 and a year later in human cells. We have therefore been working with these cells for about eight years now. As a newbie to the cardiac field, I thought by now we would be close to starting using these cells in the aforementioned areas. My previous involvement in the functional study of excitable-derived differentiated embryonic stem cells (a different type of stem cells derived from blastocysts and not adults) gave me the opportunity to know about progress being done at the time (roughly 2008) injecting yet other types of stem cells like bone marrow or human dental pulp cells into infarcted mice 4. These studies led me to think researchers were working in the right direction. Unfortunately, there seem to be some issues (mainly two) for which no answers are in place yet, according to Professor Cesare Terracciano from Imperial College London.
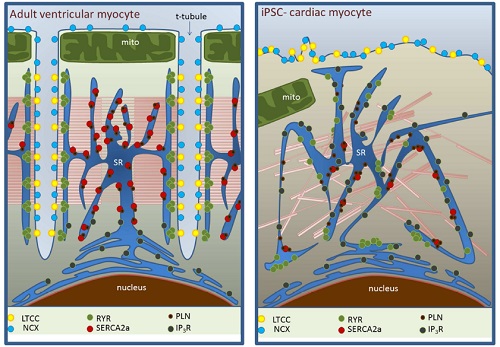
The first one relates to the elaborate intracellular organization of CM allowing them to accomplish one of their most fundamental properties, the Excitation-Contraction (EC) coupling, which transforms rhythmical electrical stimulus into mechanical forces. Most cardiac diseases have in common a dysregulation in EC coupling, thus impairing the way blood is pumped throughout the body. The iPSCs-CMs do not fully resemble the internal organization of CM, being one of the problems the absence of T-tubules, a key EC-coupling related component (fig. 2). This structure allows the entry of Ca2+ cations into the cytosol through the selective calcium channel Cav1.2. Because T-tubules are intimately apposed to yet another key structure within the cytosol called the sarcoplasmic reticulum (SR), it means that SR is highly inhomogeneous in these cells (fig. 2). Given that SR is involved in releasing and reuptaking the Ca2+ necessary for the contraction of the cell using as cue the Ca2+ ions which enter through the T-tubule, it suggests that iPSC-CM could potentially cause of arrhythmias as a consequence of an impairment in Ca2+ handling. Indeed, failing cardiomyocytes show similarities in terms of structural deficiencies. Therefore, it does not seem like the best approach to improve cardiac malfunction.
The second problem relates to the way we classify iPSC-CM as a chamber-specific population of cells. The heart contains three main types of cells with different electrophysiological properties. The atrial, ventricular and pacemaking cells. Each of these cells shows differences in their action potential (AP) shape as a consequence of a different ion channel expression profile and this is important for the correct function of the heart as a whole. Thus, most research groups claim to produce chamber-specific CM based on the electrical properties (AP morphology) of the cells. Recently, Terraciano´s group has shown that this functional classification might be inappropriate because the properties of the AP depends on how these cells are cultured in vitro (plastic or glass wells containing medium with nutrients to grow the stem cells) 5. In their study, they plated the same iPSC-CM in the same culturing conditions but just using different densities. In one group, they plated cells sparsely at a density of 15000 cells/well while a second group was plated at confluence meaning the well is full of cells (60000 cells/well). They found that the AP duration (the length from the upstroke to complete repolarization) from CM in the sparse culture was significantly longer and more heterogeneous than the ones recorded from confluent cells. That would suggest that both types of cells are different, but they are not so the density promotes changes in their functional properties. To further confirm this point and exclude the effect of intercellular communication in the confluent culture, they used carbenoxolone. This drug inhibits gap junctions, a type of channels that allow electrical coupling between different cells, essential in synchronizing the activity of CM in the whole heart. Even in the presence of carbenoxolone the difference in AP duration was maintained and although the heterogeneity was larger, this increase was small compared to sparsely plated cells. Thus, intercellular connection contribution is small and it does not explain the observed differences.
In conclusion, although remarkable progress has been done with regard to iPSC-CM differentiation, we need to find ways to obtain cells resembling the internal morphology of real CM implying a further need to improve the “recipes” currently in use. It is possible that even a completely different approach must be undertaken like applying mechanical pressure to these cells as some studies show a better differentiation profile in such conditions. Finally, different ways to classify the cells should be implemented like looking for specific molecular markers differentially expressed in each type of cell.
References
- Takahashi,K.,and Yamanaka,S.(2006).Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676. doi:10.1016/j.cell.2006.07.024. ↩
- Tomaselli GF, Zipes DP (2004) What causes sudden death in heart failure? Circ Res. Oct 15;95(8):754-63. Review. ↩
- Kane C, Couch L, Terracciano CM. (2015) Excitation-contraction coupling of human induced pluripotent stem-cell-derived cardiomyocytes. Front Cell Dev Biol. 2015 Sep 29;3:59. Review. ↩
- Gandia C, Armiñan A, García-Verdugo JM, Lledó E, Ruiz A, Miñana MD, Sanchez-Torrijos J, Payá R, Mirabet V, Carbonell-Uberos F, Llop M, Montero JA, Sepúlveda P. (2008) Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells. Mar;26(3):638-45. ↩
- Kane C, Du DT, Hellen N, Terracciano CM. (2016) The Fallacy of Assigning Chamber Specificity to iPSC Cardiac Myocytes from Action Potential Morphology. Biophys J. 2016 Jan 5;110(1):281-3. doi: 10.1016/j.bpj.2015.08.052. ↩
2 comments
[…] La investigación en células madre ha experimentado un auge considerable desde que en 2006 se descubrió un método para conseguir células madre pluripotentes a partir de células somáticas adultas. Sin embargo, hay un tipo de células en el que […]
[…] La investigación en células madre ha experimentado un auge considerable desde que en 2006 se descubrió un método para conseguir células madre pluripotentes a partir de células somáticas adultas. Sin embargo, hay un tipo de células en el que […]