Daily aspirin linked to lower cancer deaths
A study published last year in The Lancet showed that a daily dose of aspirin reduces the risk of dying from several types of cancer. According to the authors’ data, long-term daily intake reduces the probability of dying from solid tumours by more than 30%. These results are consistent with known effects of aspirin blocking cancer-related chronic inflammation in lab animal models and in some common human tumours.
Common anti-inflammatory drugs like aspirin or ibuprofen have a broad spectrum of effects. These effects are often mediated by blocking the cyclooxygenase 1 and 2 enzymes (COX-1 and COX-2), which play a major role in the affected tissue’s inflammatory response.
Inflammation is a complex physiological process that mediates infection and wound healing. However, sustained inflammation, usually referred to as chronic, can lead to tissue damage and, in some cases, is associated with tumour initiation 1. Studies on rodents indicate that aspirin and other anti-inflammatory drugs reduce incidence -this means frequency- and growth of several types of tumours. These effects are, at least in part, mediated by the inhibition of COX 2 3.
Animal models are useful to understand the basis of many diseases, including cancer, but positive results in the lab often fail to be directly translated into actually curing human diseases. Take human tumours, for example. Even specific tumours like colon cancer harbour many different mutated genes. Tumours in lab animals often lack such complexity. This is in part because they are induced by modification of one or just a few genes that are relevant for this specific tumour. So, what happens in human tumours? Studies on human colon, oesophagus and skin cancers indicate that indeed COX-2 is highly increased in the tumour tissue [2]. But are such abnormal COX-2 levels initiating the human tumours or are they just a side effect of this process?
In 2007 a group of scientists led by Dr. Andrew T. Chan published a key study in the prestigious New England Journal of Medicine on the effects of aspirin on COX-2 in the context of colon cancer 4. The researchers analysed colon cancer with increased COX-2 levels and found that its frequency was reduced among regular aspirin users. The authors concluded that regular use of aspirin seemed to reduce the incidence of a subset of colon cancer tumours that lead to high levels of COX-2. 67% of all colon cancers belong to this group, and their incidence was reduced by 34% among the regular aspirin users. Notably, the results of this study were backed up afterwards by an analysis of other aspirin trials 5.
If aspirin reduces the incidence of COX-2-associated colon cancer and COX-2 is elevated in several types of cancer, does aspirin prevent or reduce deaths resulting from other tumours? Last year a group of researches led by Prof Peter M. Rothwell set a milestone regarding the importance of the aspirin in cancer prevention that sheds light on this question 6.
Prof Rothwell and colleagues approached this problem from a very intelligent angle. Instead of running a new trial on the effects of aspirin in different tumours they decided to analyse publicly available data from previous well-conducted clinical trails with a twist. They collected data from eight different clinical trails on aspirin, which covered more than 25.000 patients in total. But none of these trails was conducted to investigate the role of this drug in cancer; they had focused instead on its preventive effects on different disorders such diabetes.
How could these studies be connected to cancer? The key is that long-term clinical trails often require detailed documentation of the subjects’ health, including causes of death -if any- even if they are not related to that particular study or occur many years after the trial started. Thus, those clinical trials, which were designed to investigate aspirin’s other potential benefits, did contain valuable data about death rates resulting from cancer.
In their study published in The Lancet, the authors show that in long-term daily aspirin users in the analysed trials, the chances of dying from cancer decrease by around 30-40%. The data was consistent across all age groups and the different trials. As the authors themselves indicate, this is quite important because the analysis of huge amounts of data often produces positive results in a few subgroups that are mere statistical artefacts and not meaningful data.
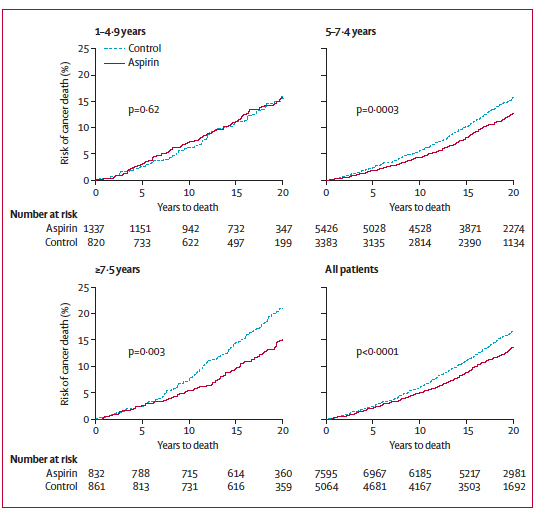
Long-term intake is defined here as more than 5 consecutive years. Subjects with shorter intake periods did not significantly benefit from aspirin, while subjects that took aspirin for more than 7.5 years benefited the most (Figure 2). The authors note that daily intake might be fundamental for the positive effect. Alternate-day aspirin trials have indeed been conducted in order to avoid some of the side effects of daily intake of aspirin, but did not show significant improvement on cancer prevention. Interestingly, the minimum daily dose of aspirin required for such striking effects was 75 mg, which is roughly the amount of active ingredient found in one children’s aspirin.
Data presented by the authors indicates that survival of most of the analysed solid cancers was increased by daily aspirin intake. Among the analysed tumours, adenocarcinomas were most susceptible to the overall benefits of aspirin use. Adenocarcinomas are tumours that originate in the glandular tissue and can be found in the lung or skin, among other organs. The authors indicate that, although data is consistent with aspirin blocking COX-2, further research may shed light on other potential mediators.
Prof Rothwell and colleagues made a great effort to ensure that their analysis was indeed meaningful and not biased or caused by other unknown effectors. As they point out, one of the strengths of their study is that the original trials were not conducted to analyse cancer. This may sound strange but, as they indicate, this avoids bias in data collection. Because the researchers involved in the original study were not interested in measuring cancer, it is unlikely the cancer-associated deaths were manipulated. Moreover authors provide a side-by-side study of aspirin together with Warfarin, another drug with some similar side effects. Warfarin intake shows no significant decrease in deaths by cancer in this study. Why this is important? The authors indicate that side effects of aspirin such as bleeding may lead to an early diagnosis of the tumours and thus reduce the death rate in this group. But because Warfarin has similar effects, yet no decline in the death rate was observed, they conclude that this could not be the cause.
According to the authors the benefits of long-term aspirin intake by far compensate negative side effects like the risk of non-fatal bleeding. Prior to this study, assessment balance of risks and benefits was based on the described increased bleeding and the lower incidence of blockage of blood vessels (vascular ischemia), respectively.
Due to the significant effect of aspirin on cancer, which is one of the major causes of mortality in Western countries, the authors indicate that the benefits of daily aspirin use highly exceed the risks. According to the authors, daily intake of aspirin for 5 to 10 years “would reduce all-cause mortality […] during that time about 10%”. This exceeds the effects of many other preventive approaches that are currently applied in most Western health systems, including breast or prostate screening.
However, this kind of studies has to be taken with some caution. There is no such thing as a silver bullet against cancer, but the quality of the trials analysed and the data presented clearly supports the authors’ claims. Long-term daily aspirin intake seems to have huge benefits in preventing death from cancer, probably by reducing its incidence. Future research may shed light on these effects in specific tumour subsets and in subjects that used aspirin for even longer periods. For now, this article in The Lancet is one of the most cited papers in 2011. This means that many scientists took this research seriously and we probably will hear more about the pros and cons of aspirin, a centenary drug that is still full of surprises.
Acknowledments:
I want to thank Dr. Bettina Trüb and César Tomé for suggestions and proof-reading.
References
- Shacter E, Weitzman SA (2002) Chronic inflammation and cancer. Oncology (Williston Park) 16: 217-226, 229; discussion 230-212 ↩
- Fosslien E (2000) Molecular pathology of cyclooxygenase-2 in neoplasia. Ann Clin Lab Sci 30: 3-21 ↩
- Williams CS, Tsujii M, Reese J, Dey SK, DuBois RN (2000) Host cyclooxygenase-2 modulates carcinoma growth. J Clin Invest 105: 1589-1594 ↩
- Chan AT, Ogino S, Fuchs CS (2007) Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med 356: 2131-2142 ↩
- Flossmann E, Rothwell PM (2007) Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet 369: 1603-1613 ↩
- Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW (2011) Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet 377: 31-41 ↩