Life and deeds of RNA (III): RNA processing, neurodegeneration and a rare disease

One of the most surprising and fascinating facts about scientific research is that one never truly knows which directions investigations may take. Along the way that goes from gathering data, to the path that links our initial question and the answer we pursue, we find numerous, intriguing and unexpected little ramifications and hideous sideways that lead to other surprising goals. The story of Lafora disease, a rare, progressive and neurodegenerative kind of epilepsy, is full of these little paths that have drawn a very curious map in which several cellular processes combine and end up in a pathological condition characterized by accumulation of aggregates in different cell types, but noteworthy, in neurons. Unluckily, to date we are still unable to link all the different molecular findings in order to find a cure. But on our way we find interesting and revealing data about processes that underlie so many other diseases, helping in the fight against metabolic disorders, epilepsies and neurodegenerative pathologies associated with abnormal removal of neuronal aggregates.
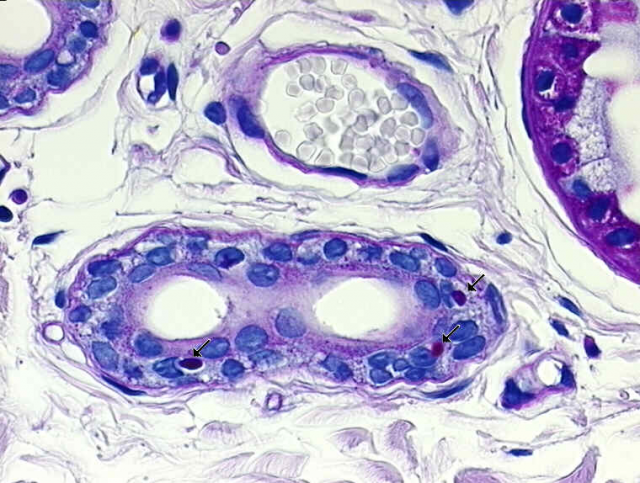
The first symptoms of Lafora disease take place in adolescence, with a sudden epileptic crisis, which in turn becomes more and more frequent and is quickly followed up by a series of terrible neurodegenerative issues that lead to visual loss and even dementia, among others. Finally, in no more than 10 years since the first crises, the patient dies. To date, only palliative treatments are available and a cure seems far to be reached, however in the last twenty years many and very interesting molecular details of what is going on in the nervous system of those affected by Lafora disease have been uncovered. Very briefly, we now know that neurons, as well as other tissues, accumulate strange aggregates composed in a vast majority by an abnormal form of glycogen (these aggregates were named Lafora bodies; Figure 2); since neurons do not even use glycogen in their normal life, it was soon assumed that glycogen metabolism had something to do with this weird chain of events. The genomic era has provided us of tools that define which genes are involved in this rare hereditary pathology, and curiously it appeared that there were two genes involved, one coding for a protein with phosphatase activity (usually a task related to switching on and/or off molecular signaling events), called laforin, and a protein with ubiquitin ligase activity (involved in the labeling of proteins in order to mark them for different purposes, very often for degradation), called malin. Although biochemically and structurally they are strikingly different, both are able to interact and form a commando that regulates glycogen metabolism. At the precise moment that this was found, it seemed that the explanation to Lafora disease was almost complete, but things in biology are always much more complicated. Laforin and malin are also involved in the control of cellular stress produced by the accumulation of unfolded proteins (a topic reviewed in Mapping Ignorance, here and here). Now the landscape became enriched with several new data, and with the work we review today, it suffers an even more interesting twist. And of course, following the central theme of this series, it has to do with RNA.
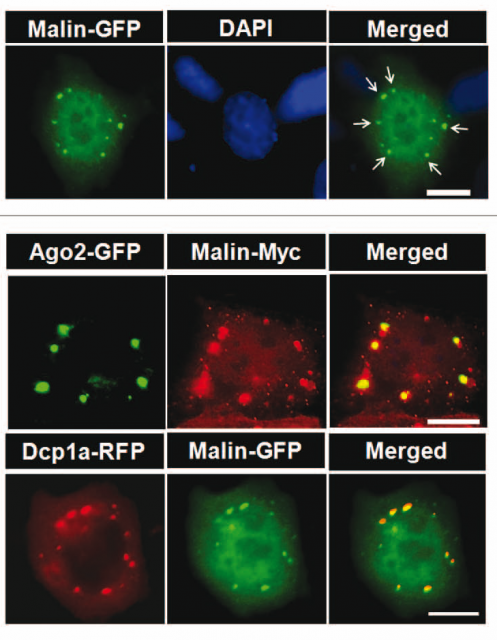
The group of Subramaniam Ganesh, with a long trajectory in the study of Lafora disease, has recently published a very intriguing work describing the participation of the protein malin in the processing of RNA 1. Sweta Singh and collaborators, guided by the observation that malin location inside the cell significantly varied when applying stimuli that specifically promoted the increase in processing bodies (in the last issue we learnt that processing bodies are multi-protein complexes involved in RNA modification), they found that malin was indeed attached to processing bodies (Figure 3) and specifically bound to a protein named Dcp1a, with a critical role in microRNA processing. Several experiments confirmed that the union between Dcp1a and malin was specific, since although the location was the same, no other proteins from the complex were interacting with malin (remember the protein named Argonaute?); and furthermore, malin displayed the capability of promoting the ubiquitination of Dcp1a. This, in a simpler way, means that Dcp1a is labeled for degradation and disappears from the cell, with the consequence that several microRNAs are stopped on their way towards their destination. The most striking consequence of this interference of malin with the RNA processing system is that it severely affects the activity of Dicer, again, a critical protein in the final steps of microRNA-mediated gene silencing. This findings are by themselves interesting, but the physiological confirmation makes them even more robust: mice deficient in the malin gene showed a reduction in the levels of Dcp1a and, as expected, of Dicer: physiological treats that have previously been related with neurodegenerative processes like those observed in Parkinson’s, Alzheimer’s or Huntington’s disease. Also seizures and ataxia have been reported to be related to these molecular alterations in mice, and as we said at the beginning of the text, they are typical symptoms observed in Lafora disease.
The conclusions of the work are fascinating in several aspects. First, they show how intriguing the paths of basic research initiatives can result. For long years glycogen metabolism has guided research in Lafora disease, but in the very latest, many other cellular processes are starting to appear, highlighting the crosstalk and interaction between diverse and apparently unrelated processes. A complex problem like that involving neurodegenerative processes can only be addressed looking in many different directions. Also, we have here another nice example of how RNA processing and regulation reveals as a powerful tool, but also as a precisely controlled and versatile process upon which critical tasks are committed in living cells. Maybe the most singular features of many neurodegenerative diseases, like the amyloid plaques in Alzheimer’s, or the Lafora bodies in Lafora disease, which have been the focus of research for so long, are just consequences of preliminary and much more subtle alterations that we are starting to perceive. Probably all these factors have to be taken into account, but the determination of the timing of events, and the knowledge of which process could be stopped or slowed down in order to limit the progression of the disease, may be just around the corner. Will the old folk RNA, so often considered like the most primitive macromolecule (and probably the first catalyst that made possible the pass from inorganic matter to organic life), hold the key for the cure of complex diseases in an extremely evolved nervous system? It would be a very strange and amazing paradox. But when it comes to life matters, in the end, nothing is odd enough as not being possible.
References
- Singh S., Singh P.K., Bhadauriya P. & Ganesh S. (2012). Lafora disease E3 ubiquitin ligase malin is recruited to the processing bodies and regulates the microRNA-mediated gene silencing process via the decapping enzyme Dcp1a, RNA Biology, 9 (12) 1440-1449. DOI: 10.4161/rna.22708 ↩
2 comments
I have learned so much from reading your blog. thank you.
Thank you for reading! I’m glad it was useful for you. Cheers!