Using mass spectrometry to measure circulating histones against sepsis progression
Interior of a human blood vessel. Something looks wrong, very wrong. Bacteria have entered the circulation, and the immunological system is reacting. All the alarms are on fire, while an army of white blood cells comes by, getting ready to unleash hell on the invaders. Suddenly, among these security agents, a neutrophil stands out. It is huge. It seems unstable. And suddenly, without further warning, it attacks the invaders by an unexpected strategy.
It kills itself. And instead of unleashing hell… it unleashes its nuclear contents.
DNA, proteins and a mesh of chromatin burst out into the blood and adhere to the cell walls of the surprised bacteria. They cannot escape from this molecular trap, and they serve as bait for more and more sister cells, which get also stuck into the mortal mesh. And then, the proteins that used to be the vital scaffold for that neutrophil’s own genetic material – the histone proteins around which DNA strands orderly wrapped themselves – are loose on the mayhem. Their highly positive “tails” of basic amino acids are glued with the negative charges of the bacterial lipopolysaccharide cell walls, thus contributing to the mortal trap. Now, the macrophages will have an easy task to get rid of these unwanted visitors, with no need to keep running around the circulation. Dinner’s served. And the battle will speed up towards a happy ending for the host.
But… will it, really? Let’s wait and see.
Some histones get off the traps. They bind to the endothelium, the sheath of specialized cells in the inner side of the vessels. And then, catastrophe ensues. Because the capacity of histones to kill bacteria does not only rely on their formation of traps: histones are a trap themselves. They are cytotoxic. The contact with cell membranes triggers mechanisms that will lead to the cell’s death. And remember, inside your body, there are no good and bad guys; there is no purpose, just automatism. So any histones that touch your own cells, will kill them. And then, in turn, those dead cells with compromised membranes will also release their content to the bloodstream. And, among other million things, cells usually contain… histones. What was initiated as a defence mechanism against an invader, suddenly results in a vicious circle in which the trap has produced a chain reaction. If the situation lasts for too long, the damage might extend to the entire body through the highways of blood vessels.
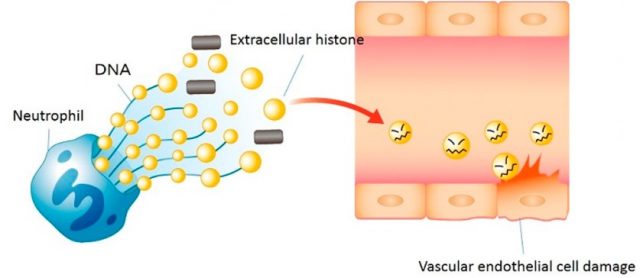
This scene we have dramatically depicted is based on the reactions taking place, at a molecular level, inside the bloodstream of a person undergoing a septic process. The terrible effects of sepsis, initiated by an infection, are mostly due to the prolonged and, in turn, deregulated response of the patient’s immunological system to that infection. And among many different compromised systems, that of NETosis (the formation of the so called Neutrophil Extracellular Traps that involves the release of nuclear material) is the one that could, in turn, fill the blood vessels with histone proteins. It is quite surprising that those tiny proteins, related to the maintenance of DNA structure and the fine-tuning of gene expression (find more about them in all these articles), can turn into a hydeish version once out of their original environment inside the cell nucleus. This “dark side” of histones 1 has been known since the fifties of the last century, thanks to the pioneer discoveries of Hirsch 2; surprisingly enough, the specific mechanisms underlying such a dangerous behavior are still object of debate and thorough investigation for many groups, including ours 3, throughout the world. We keep struggling in the determination of the molecular pathways and cellular processes that lead to this race of death, in the hope of discovering slight details that may be of help in minimizing the terrible effects of histone release into the circulation.
But in the meanwhile, and as many other times before in the history of biomedicine, we are learning to use the facts for addressing the problem from a practical perspective: the mere presence of histones can be used to diagnose diseases. And in the case of sepsis, the specific amount of histones in the blood could predict the outcome of patients, since histones levels are increased the longer the process is sustained. In the complex landscape of sepsis, we find overwhelming diversity between patients, being very difficult to anticipate if, or when, they will undergo septic shock (kind of a next-level sepsis in which multi-organ dysfunction is almost beyond repair). Together with the lack of specificity of treatments until the real focus of infection is found, clinicians find themselves in a very tough situation, having to take hard and sometimes risky decisions and using medical resources which can be quite aggressive, not to say scarce, in too many countries. Thus, the measuring of histones in blood has been earlier proposed as a good measure to predict the fatal outcome of patients and a prognostic parameter in order to initiate specific protocols of action.
The only problem is that measuring histones is not easy; plasma samples obtained from the blood of patients are complex matrices full of very different proteins, and methods based in immunodetection (recognition by antibodies) show low reproducibility and high error rate. When years ago my colleague José Luis García-Giménez, working in the group of Federico Pallardó at the University of Valencia, first told me about this “dark side of histones”, I could not imagine I would end up working side by side with them, developing a detection method based on the accurate and specific technology of mass spectrometry.
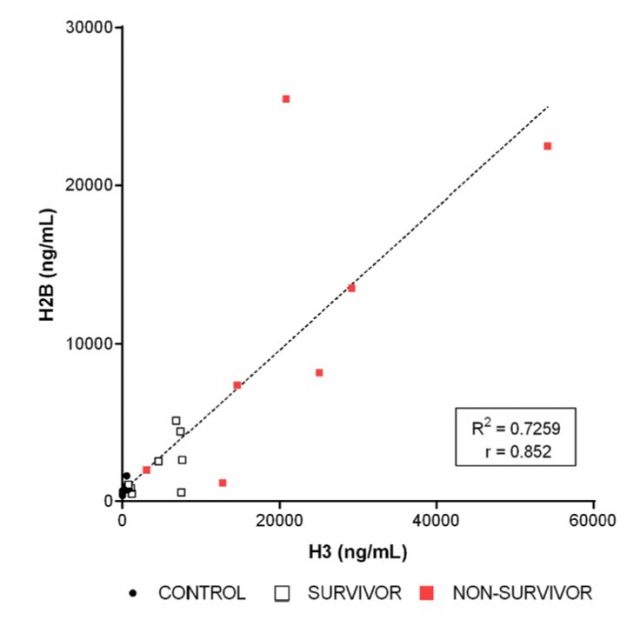
This proteomic method is known by its capacity to identify proteins in complex environments, distinguishing among them at the amino acid sequence level. But there are variations of the technique that permit also the quantification of specific proteins, by using a simple trick: addition of an internal standard, a specific peptide mimicking one of the histones, but isotopically labelled. When reading the results of the mass spectrometry run, we will find some pairs of peaks in the spectrum, very close together: the heavy (labelled) and the light ones (from the biological sample). Because we know exactly which amount of heavy peptide was added, we can estimate the amount in the original sample and, from there, mathematically calculate the relative amount of histone in the blood of the patients. Obviously, the method is complex (it uses a variant of targeted mass spectrometry called Multiple Reaction Monitoring, or MRM-MS) and involves a lot of previous work (selection of the most accurate peptide that shows strong linearity between detection levels and real concentration, for starters). But when we used it to compare samples from healthy individuals (ideally with very few, or no histones at all circulating in their blood) and septic shock patients, we could see that patients showed strikingly high levels of histones; but the most interesting data was the observation that the highest histone levels were always registered in those patients that, sadly, in the end, did not survive the septic shock episode. On the other hand, surviving patients showed lower levels of histones.
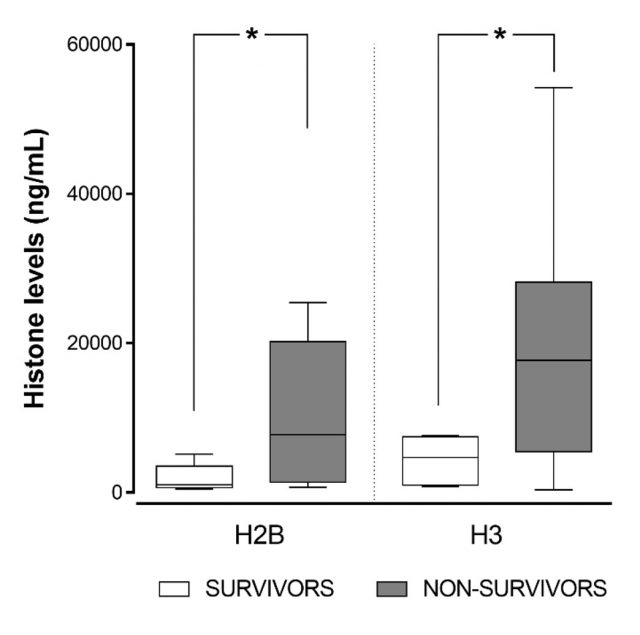
These results are preliminary, and our group of patients is still small. But we are studying more, and we are using the method to measure other histones and related proteins. The use of different and more accurate controls will also strengthen our method; but at the moment, you can take a look at how these data seem quite promising, as we have just published in Scientific Reports 4. We aim to transform these data into a practical tool that permits clinicians to take rapid and efficient measures in specific patients; because, in sepsis, they live a race against time. They fight with a faceless enemy, disguised upon unspecific symptoms and offering no clues of where to hit next. If we manage to unveil the molecular determinants of histone-mediated injury, and how different amounts of histones in blood correlate with progression towards one direction or another (some patients undergo a sudden septic shock, others start with disseminated intravascular coagulation… their needs and fates are totally different), we will facilitate the taking of otherwise drastic and critical decisions. We are still far from a direct algorithm of actions; but now, at least, we have a powerful tool, not only to detect the presence of histones but to measure them in a very precise and reliable way. It might be true that histone proteins can fall into the Dark Side, but they can also illuminate the way to escape the traps of our own immunological system.
Only that instead of blindly trusting the Force… we may better use mass spectrometry.
References
- Chaput C, Zychlinsky A. Sepsis: the dark side of histones. Nat Med. 2009 Nov;15(11):1245-6. doi: 10.1038/nm1109-1245. PubMed PMID: 19893552. ↩
- Hirsch JG. Bactericidal action of histone. J Exp Med. 1958 Dec 1;108(6):925-44. PubMed PMID: 13598820; PubMed Central PMCID: PMC2136925. ↩
- Pérez-Cremades D, Bueno-Betí C, García-Giménez JL, Ibañez-Cabellos JS, Hermenegildo C, Pallardó FV, Novella S. Extracellular histones disarrange vasoactive mediators release through a COX-NOS interaction in human endothelial cells. J Cell Mol Med. 2017 Aug;21(8):1584-1592. doi: 10.1111/jcmm.13088. Epub 2017 Feb 28. PubMed PMID: 28244682; PubMed Central PMCID: PMC5543457. ↩
- García-Giménez JL, Romá-Mateo C, Carbonell N, Palacios L, Peiró-Chova L, García-López E, García-Simón M, Lahuerta R, Gimenez-Garzó C, Berenguer-Pascual E, Mora MI, Valero ML, Alpízar A, Corrales FJ, Blanquer J, Pallardó FV. A new mass spectrometry-based method for the quantification of histones in plasma from septic shock patients. Sci Rep. 2017 Sep 6;7(1):10643. doi: 10.1038/s41598-017-10830-z. ↩
1 comment
[…] Las potencialmente catastróficas consecuencias de la sepsis, que se inicia por una infección, vienen provocadas por el propio sistema inmune que la combate perdiendo el control y entrando en un cículo vicioso. Carlos Romá-Mateo en Using mass spectrometry to […]