Grasshopper mouse mighty powers against evil bark scorpion: a molecular tale
Natural selection has been sculpturing living organisms for millions of years, enabling them to get adapted to an ever-changing environment. Gradually, certain traits are selected over others based on the advantage they confer to the population. Natural selection is not a random process and it relies on genetic variation. Random mutations serve as the substrate that ultimately leads to adaptation. However, not all traits are equally susceptible to change because some of them play an important role for the survival of the individual. Thus, mutations affecting these characteristics may not be selected, as it may be the case on genes expressed during embryonic development.
A similar obstacle is found when mutations emerge in genes related to pain sensitivity, mainly because survival prospects of individuals rely upon sensing of harmful stimuli to detect and avoid tissue damage. Accordingly, mutations affecting pain pathways are seldom in the animal kingdom (at least in high mammals). In the last decade, we have identified few genes in patients (and consequently, proteins) involved in sensing pain, thanks to state of the art sequencing techniques and medical doctors. Occasionally, the consequences for those individuals are calamitous because the lack of warning systems makes them fearless to potentially fatal circumstances. This is the sad case of a boy from Lahore (Pakistan) who wanted to impress his friends on his birthday. He decided to jump off the first floor roof of his house. He was apparently fine, but died a day later because of haemorrhage. He had a rare disease called congenital pain insensitivity that means he had never experienced pain.
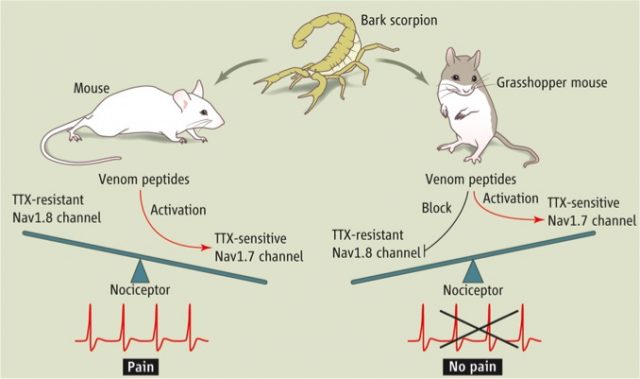
That’s the reason why a recent publication on Science journal by Rowe et al. has drawn attention in the field1. Grasshopper mice (Onychomys spp.) inhabit dry, sandy areas like deserts with minimal vegetation. It is a bulkier rodent with a greyish to pale brown fur on top and white underbelly. The striking finding comes from the stuff they like to eat. Bark scorpions (Centruroides spp.) are an important element of their diet; the problem is they don’t like to be eaten, inflicting painful stings that may last up to several hours. The question would then be whether grasshopper mice are skilful enough to avoid being stung or rather they have some kind of physiological resistance to the pain caused by the toxin injected through the sting. Surprisingly, observations from a feeding trial showed that they responded to the scorpion’s sting by briefly grooming before attacking and consuming them2.

The authors began exploring if pain sensitivity from the grasshopper mice was somehow affected by using the formalin test, a widely used pain model that produces both acute and inflammatory pain in laboratory mice. It consists of an injection of an aqueous solution of the chemical compound formaldehyde to the paw of the mice. After injection, mice show an early pain response (they start licking their paw) as a consequence of the direct action of formaldehyde on sensory fibres (called nociceptors). This initial period is known as acute phase. The early phase is followed by a more sustained one (lasting for 30 minutes roughly) and reflects an inflammatory reaction. After formalin injection, both Mus musculus (the most widely mice used in research) and Onychomys torridus licked their paws, although the grasshopper mice did it less than M. musculus, indicating that both mice respond to both acute and inflammatory pain.
Rowe et al. then isolated and collected DRG neurons from both mice and plated them in petri dish. Activation of the fresh isolated neurons by artificially depolarizing them led to action potential firing in both cases, in the absence of the scorpion’s venom. When the toxin was applied to the culture, only the grasshopper mice neurons showed a complete block of the action potential firing. Notably, both types of neurons were depolarized after current injection. This result gave them an important clue about what was happening.
Sensory neurons express two different voltage-gated sodium channels important in conveying information about pain to the central nervous system (CNS). They are called Nav1.7 and Nav1.8. While Nav1.7 is important to initiate action potential in DRG by depolarizing them, Nav1.8 propagates the action potentials to the CNS. Therefore, their result from isolated neurons suggested that both types of neurons express “normal” Nav1.7 ion channels that get activated by the toxin (in both cases the response was initiated) while Nav1.8 function was impaired only in the grasshopper mice, because propagation of the pain signal was abolished (the action potential firing). Given that bark scorpion venom is known to activate Nav1.7, this result was quite odd because it suggested that while Nav1.7 is activated, the same toxin inhibits Nav1.8 in the grasshopper mice.
To identify the region of Nav1.8 involved in the venom-mediated inhibition, the authors carried out a series of elegant genetic engineering work by creating chimeric channels, which are composed of the modules of different channels. Nav channels consist of four homologous domains named I to IV (subunits or monomers) bound by cytoplasmic tails; so they created an engineered channel were each domain of the grasshopper mice Nav1.8 channel was replaced by the corresponding domain of the ‘common’ Nav1.8 version (the one from Mus musculus). This approach allowed them to identify domain II as containing the relevant molecular determinants for the block as shown by the lack of inhibition on this chimera.
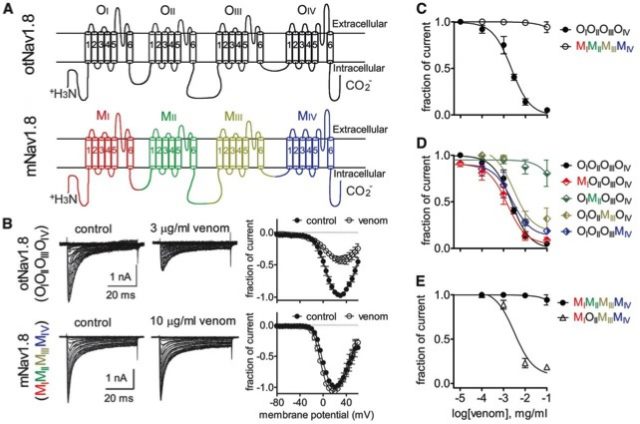
Site directed mutagenesis was then used to narrow down the region from subunit II accounting for the neurotoxin-mediated inhibition. Amino acids previously known to affect Nav1.8 activity by other scorpion peptide venom were mutated. An acidic amino acid (E862) adjacent to the pore (or gate) of the channel was found to be critical for the block, being an amino acid quite conserved among mammals. Therefore, a slight variation in the sodium channel structure produces a substantial physiological change without affecting acute pain or inflammatory responses.
Evolution has found over millions of years a strategy to circumvent the inconvenience to prey on a rather inaccessible food resource. This subtle change on a single protein converts a potent neurotoxin into a rather excellent analgesic. It seems logical to argue that the extremity of the niche to which grasshopper mice have adapted to has played an important role. For scientists this finding is significant because it confirms previous studies done under “less physiological” conditions (mainly transgenic mice studies) highlighting the importance of Nav1.8 as a relay of hazardous stimulus. In addition, it seems promising for pharmaceutical companies as it may help them to develop a new generation of analgesic drugs by mimicking what natural selection has done over time. If we can find a way to prevent pain without the undesired addiction problems associated to the most potent current treatments, as it happens with opioids that would give hope to people suffering of chronic pain conditions.
References
- Rowe A.H., Xiao Y., Rowe M.P., Cummins T.R. & Zakon H.H. Voltage-gated sodium channel in grasshopper mice defends against bark scorpion toxin., Science (New York, N.Y.), PMID: http://www.ncbi.nlm.nih.gov/pubmed/24159039 ↩
- Rowe AH, Rowe MP: Risk assessment by grasshopper mice (Onychomys spp.) feeding on neurotoxic prey (Centruroides spp.). Anim Behav 2006; 71: 725-734. ↩
2 comments
[…] ¿Cómo es posible que un ratoncito se dedique a comer escorpiones y no le pase nada a pesar de los picotazos? Sergio Laínez desentraña este misterio molecular y sus posibles aplicaciones terapéuticas en Grasshopper mouse mighty powers against evil […]
[…] ere? Sergio Laínezek misterio molekular hau eta honen aplikazio terapeutikoak argitzen dizkigu Grasshopper mouse mighty powers against evil bark scorpion: a molecular tale […]