Remodeling of the immune microenvironment in B-ALL leukemia
Author: Marta Irigoyen is a postdoctoral researcher at CIC bioGUNE
Acute B-cell progenitor acute lymphoblastic leukemia (B-ALL) is a clonal hematopoietic neoplasm characterized by the proliferation and accumulation of B-lymphoid progenitor cells throughout the bone marrow. Although important studies have characterized the underlying biological pathways that underpin therapy failure 1, resistance and subsequent relapse 23 remain a leading cause of cancer-related deaths 4. One approach to overcoming therapy resistance is to assess the role of the bone marrow microenvironment in promoting and supporting leukemia survival and progression.
A number of recent studies have characterized the immune microenvironment of human solid tumors 5, where malignant transformation coincides with extensive re-modeling of the immune microenvironment. However, how B-ALL transformation directly affects the bone marrow remains unclear yet. To address these events during leukemia emergence and progression, Witkowski and collaborators find out a role for non-classical monocytes in B-ALL survival, and demonstrate that their depletion prolongs disease remission in vivo, supporting a new immune-based therapeutic approaches for high-risk B-ALL treatment 6.
Firstly, to uncover the immune landscape of high-risk acute leukemia, authors carried out scRNA-Seq (a technique that examines the gene expression level of individual cells in a given population by simultaneously measuring the RNA concentration of hundreds to thousands of genes) of mononuclear bone marrow specimens taken from primary B-ALL patients at disease diagnosis and healthy bone marrow donors. Thus, the extensive analysis of the obtained clusters showed a significant reduction in the fraction of myeloid cells in diagnosis compared to healthy bone marrow. Besides, gene set enrichment analysis showed diagnosis B cell lineage cells significantly enriched for gene expression profiles associated with DNA repair and cell cycle progression, likely resulting from a large proportion of transformed B cell progenitors.
Given that the myeloid compartment was significantly diminished as a result of emergence of B-ALL in the bone marrow, authors next aimed to understand the specific myeloid subpopulations impacted by leukemia and identified the two myeloid cell types most significantly affected by the presence of bone marrow B-ALL as classical CD14-expressing monocytes, and non-classical CD 16-expressing monocytes. To understand functional differences between leukemia-associated non-classical monocytes and their healthy counterparts, they performed differential gene expression analysis, which revealed a leukemia-associated up-regulation of genes involved in monocyte interactions with the vascular endothelium during inflammation and vascular endothelial repair 7, such as PECAM1, CD44, ITGA4, CX3CR1 and TNFSF10.
Although authors observed extensive re-modeling of the myeloid compartment at disease diagnosis, the impact of conventional chemotherapy and disease re-emergence on leukemia-associated myeloid cells is unknown. To investigate this further, they analyzed myeloid cells and identified several clusters present in the combined diagnosis, remission and relapse bone marrow. Here, they observed a significant increase in the fraction of non-classical monocytes, throughout both diagnosis and relapse when compared to remission bone marrow. Notably, non-classical monocytes presented at diagnosis and relapse, showed and increased expression of genes involved in vascular endothelial interactions when compared to remission, including PECAM1, TNFSF10 and CX3CR1 genes.
To validate their transcriptional observations, they employed several approaches to characterize the monocytes presented in B-ALL patients at the protein level. CITE-Seq is a sequencing-based method that simultaneously quantifies cell surface protein and transcriptomic data within a single cell readout 8. Using CITE-Seq they analyzed the myeloid compartment in diagnosis, remission and relapse bone marrow mononuclear cells from primary B-ALL patients. They also used flow cytometry analysis. Interestingly, the myeloid-enriched cell subpopulation showed a significative increase of CD14dimCD16+ non-classical monocytes at both B-ALL diagnosis and relapse respect to healthy controls. Besides, consistent with their previous transcriptional data, the levels of genes involved in vascular endothelial interactions (namely PECAM1/CD31 and CD49d/ITGA4) were overexpressed in non-classical monocytes.
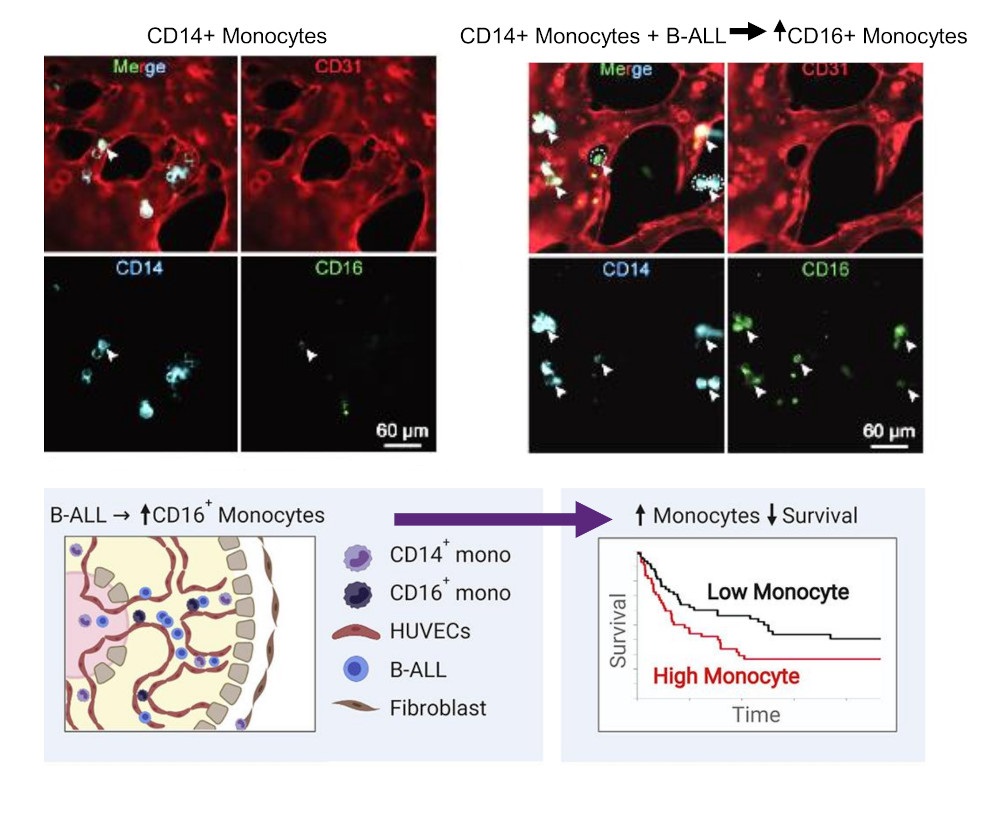
To analyze if the described non-leukemic monocytic subpopulations offer prognostic significance for B-ALL patient survival, they next assessed the impact of peripheral blood monocyte counts on B-ALL patient outcome. Thus, they took advantage of an independent cohort of pediatric newly diagnosed B-ALL patients in which peripheral blood parameters were assessed at four time points: i) initial disease diagnosis; ii) end of induction therapy; iii) initiation of maintenance therapy, and iv) end of therapy. For each of the four time points analyzed, B-ALL patients were separated on the basis of peripheral blood absolute monocyte count (AMC), generating two groups: a) patients with absolute monocytosis, AMC > 1000 cells/μL; and b) AMC ≤ 1000 cells/μL. Interestingly, B-ALL patients presenting with absolute monocytosis at disease diagnosis (i) had a significantly inferior overall survival (OS) and relapse-free survival (RFS). Having demonstrated a prognostic role for monocyte abundance in the peripheral blood of pediatric B-ALL, they sought to extend their analysis to adult B-ALL cases 9. After analysis, they noted a significantly inferior RFS and OS in adult Monocyte-high B-ALL patients when compared to adult Monocyte-low patients, further suggesting that monocytes play a protective role in tumor cell survival.
Next, they sought to define the origin of such non-classical monocytes detected in the B-ALL bone marrow. Previous studies have highlighted a critical role for vascular endothelium in promoting the differentiation of classical to non-classical monocytes 10. As they observed that leukemia-associated monocytes up-regulated multiple genes involved in vascular endothelial interactions (e.g. PECAM1/CD31), they hypothesized that B-ALL cells were able to interact with the vascular microenvironment to favor the emergence of non-classical monocytes. Thus, they generated a 3D-organotypic vascularized device using human umbilical vein endothelial cells (HUVECs) to coculture human B-ALL cells together with human CD14+ monocytes 11. Confocal microscopy analysis revealed that the seeding of human CD14+ monocytes resulted in the appearance of non-classical CD 16-expressing monocytes, and the presence of B-ALL significantly increased the percentage of human CD 16-expressing monocytes ex vivo and increased microvessel diameter.
To further validate the importance of leukemia-associated non-classical monocytes during B-ALL progression, authors took advantage of a well-characterized animal model of pediatric Philadelphia (Ph)+ B-ALL (the Ph chromosome is the most common abnormality in the leukemia cells of B-ALL patients) 12. To further understand the impact of murine B-ALL on the bone marrow and peripheral blood, they performed scRNA-Seq of two independent primary B-ALL recipient animals and two healthy littermate controls. Here, they observed an increased fraction of non-classical murine monocytes in the peripheral blood of the B-ALL recipient compared to healthy controls. Interestingly, B-ALL recipient murine non-classical monocytes also showed high Pecam1 expression relative to healthy non-classical monocytes. They then hypothesized that targeting monocyte subsets could affect B-ALL treatment responsiveness in vivo. Previous studies have demonstrated that the blockage of “Colony Stimulating Factor 1 Receptor” (CSF1R; This signaling is necessary for the survival, proliferation and differentiation of myeloid cells) prevented the maturation of non-classical monocytes in vivo 13.
Taking all this into account, they injected either anti-CSF1R or control antibodies (IgG) and, 12 days post B-ALL transplantation, they observed a depletion of leukemia-associated monocytes, including both classical monocytes and non-classical monocytes after anti-CSF1R treatment. Having established the effects of CSF1R blockade on monocytic development in vivo, they sought to test the significance of non-classical monocytes in B-ALL therapy responsiveness using the tyrosine kinase inhibitor nilotinib (a chemotherapeutic drug). Although nilotinib treatment was able to significantly prolong the survival of IgG-treated recipient survival, most of the animals eventually succumbed to B-ALL relapse. Nevertheless, the combination of anti-CSF1R and nilotinib was able to enhance the overall survival and decrease the percentage of mice succumbing to re-emerging leukemia by 70 days post-transplantation.
In conclusion, the researchers uncovered a new role for non-classical monocytes in B-ALL survival. They also demonstrated that the presence of abundant monocytes during B-ALL diagnosis is a valid predictive prognosis value for both pediatric and adult B-ALL patient survival. Besides, they showed that depleting leukemia-associated monocytes in B-ALL animal models increase animal lifespan. Moreover, the profiling of the B-ALL immune microenvironment was a valid tool to identify extrinsic regulators of B-ALL survival, supporting new immune-based therapeutic approaches for high-risk B-ALL treatments.
References
- Mullighan CG, Phillips LA, Su X, Ma J, Miller CB, Shurtleff SA, Downing J.R. Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science. 2008; 322: 1377-1380. PMID: 19039135 DOI: 10.1126/science.1164266. ↩
- Slayton WB, Schultz KR, Kairalla JA, Devidas M, Mi X, Pulsipher MA et al. Dasatinib plus intensive chemotherapy in children, adolescents, and young adults with Philadelphia chromosome-positive acute lymphoblastic leukemia: results of Children’s Oncology Group Trial AALL0622. J Clin Oncol. 2018; 36: 2306-2314. PMID: 29812996 DOI: 10.1200/JCO.2017.76.7228. ↩
- Witkowski MT, Lasry A, Carroll WL, Aifantis I. Immune-based therapies in acute leukemia. Trends Cancer. 2019; 5: 604-618. PMID: 31706508 DOI: 10.1016/j.trecan.2019.07.009. ↩
- Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015; 373: 1541-1552. PMID: 26465987 DOI: 10.1056/NEJMra1400972. ↩
- Azizi E, Carr AJ, Plitas G, Cornish AE, Konopacki C, Prabhakaran S, Nainys J et al. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell. 2018; 174: 1293-1308.e36. PMID: 29961579 DOI: 10.1016/j.cell.2018.05.060. ↩
- Witkowski MT, Dolgalev I, Evensen NA, Ma C, Chambers T, Roberts KG et al. Extensive Remodeling of the Immune Microenvironment in B Cell Acute Lymphoblastic Leukemia. Cancer Cell. 2020; 37: 867-882.e12. PMID: 32470390 DOI: 10.1016/j.ccell.2020.04.015. ↩
- Gerhardt T, Ley K. Monocyte trafficking across the vessel wall. CardiovascRes. 2015; 107: 321-330 PMID: 25990461 DOI: 10.1093/cvr/cvv147. ↩
- Stoeckius M, Hafemeister C, Stephenson W, Houck-Loomis B, Chattopadhyay P.K, Swerdlow H, Satija R, Smibert P. Simultaneous epitope and transcriptome measurement in single cells. Nat Methods. 2017; 14: 865-868. PMID: 28759029 DOI: 10.1038/nmeth.4380. ↩
- Roberts KG, Li Y, Payne-Turner D, Harvey RC, Yang YL, Pei D et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med. 2014; 371: 1005-1015. PMID: 25207766 DOI: 10.1056/NEJMoa1403088. ↩
- Gamrekelashvili J, Giagnorio R, Jussofie J, Soehnlein O, Duchene J, Briseno CG et al. Regulation of monocyte cell fate by blood vessels mediated by Notch signalling. Nat Commun. 2016; 7: 12597. PMID: 27576369 DOI: 10.1038/ncomms12597. ↩
- Boussommier-Calleja A, Atiyas Y, Haase K, Headley M, Lewis C, Kamm RD. The effects of monocytes on tumor cell extravasation in a 3D vascularized microfluidic model. Biomaterials. 2019; 198: 180-193. PMID: 29548546 DOI: 10.1016/j.biomaterials.2018.03.005. ↩
- Li S, Ilaria Jr., R.L, Million R.P, Daley G.Q, Van Etten R.A. The P190, P210, and P230 forms of the BCR/ABL oncogene induce a similar chronic myeloid leukemia-like syndrome in mice but have different lymphoid leukemogenic activity. J Exp Med 1999; 189: 1399-1412. PMID: 10224280 DOI: 10.1084/jem.189.9.1399. ↩
- MacDonald KP, Palmer JS, Cronau S, Seppanen E, Olver S, Raffelt NC et al. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood. 2010; 116: 3955-3963. PMID: 20682855 DOI: 10.1182/blood-2010-02-266296. ↩