Nanoscale mixing: the case of titanosilicates
Author: Andi Cuko is a Ph.D. student (ITN-EJD-TCCM) at Université Pierre et Marie Curie
Oxides are inorganic compounds that contains at least one oxygen atom which is bounded to one or more other elements and they are the most abundant solids in the earth crust. In our society they are playing a very important role in technological field as diverse as catalysis, photovoltaics and electronics. They have been used so successfully due to the combined high chemical stability with wide range of chemical and physical properties. In last years, oxides materials but not only, have been used in some cases as tiny particles at nanoscale size (1nm = billionth of one metre). It is known that when the size of a certain material is reduced at nanoscale it starts to display specific non-bulk-like properties. Such nanomaterials, are attracting a great deal of attention and they find more and more applications in emerging nanotechnologies. In this article we are going to focus on one particular case, mixed TiO2-SiO2.
Materials based on mixing titania and silica, titanosilicates are technologically important for many applications such as industrial catalyst, solar cells, self cleaning systems, selective molecular sieves, materials for removing water pollutants and photocatalysts for many reactions 123. In each case it is the interfaced combination of the two oxide nanophases that determines the properties of the final nanostructure.
On the one hand, titania (TiO2) exhibit exceptional electronic properties such as photoactivity which strongly depends on the size of the nanoparticles. In nature, titanium dioxide can be found mainly as three polymorphs: rutile, anatase and brookite. Rutile is most thermodynamically stable phase at ambient conditions while anatase is most photoactive polymorph. For instance, anatase nanoparticles are able to split water in H2 and O2 when irradiated with UV-light (Degussa P25 catalyst), or they can be used as self cleaning hydrophobic coating to apply on glasses or on building walls, in this way, the organic molecules forming the dirtiness are oxidized to form CO2 just after their deposition in the presence of solar light.
On the other hand, there is silica (SiO2) which is an insulator with highly chemical, thermal and mechanical stability. Silica is one of the most complex and most abundant families of materials, existing both as several minerals and being produced synthetically. Applications range from structural materials to microelectronics to components used in the food industry. By mixing SiO2 with TiO2 it is possible to obtain a material with improved physico-chemical properties. For instance, by using a microporous silica-based material which guarantees a very high surface area with anatase nanoparticles embedded in silica pore wall it is possible to achieve higher photocatalytic performances than the pure titania.
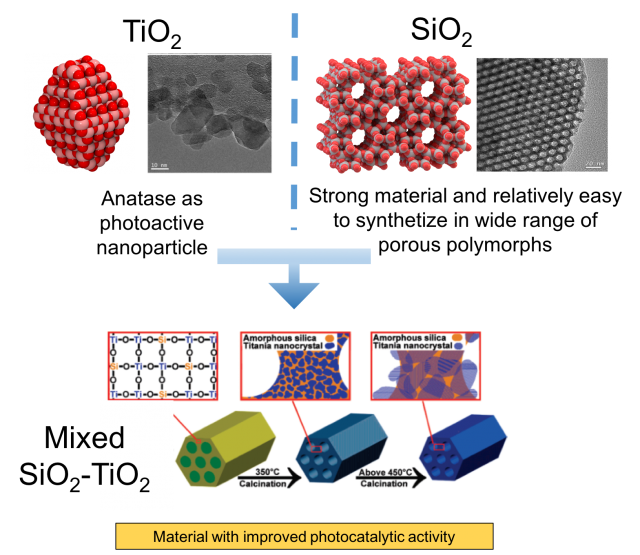
Another example of mixed SiO2-TiO2 materials is the titanosilicate-1 (TS-1), known from early 80s. It is non photoactive very important catalyst used in industry for the oxidation of organic molecules in mild conditions with the presence of hydrogen peroxide. Its structure, shown in Figure 2, is characterized by a zeolite like silica framework (MFI family) with titanium oxide as active center on the micropore surfaces. Titanosilicates although clearly constitute an important nano-oxide composite system, relatively little is known about the nature and role of the interface in these systems.
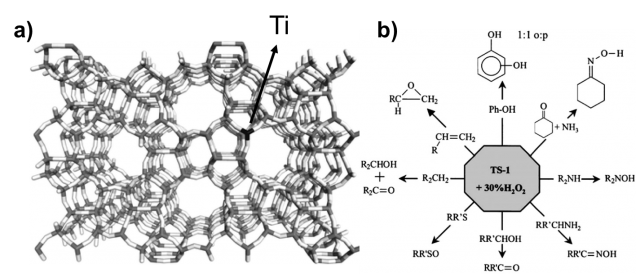
Understanding the structure-reactivity of these systems is crucial in order to improve or even design new and more efficient mixed titania-silica materials. Here we use a range of computational modelling methods in order to understand the driving force behind the mixing of titania with silica at microscopic level. The primary goal is to understand why and how these materials mix together at the nanoscale. The secondary objective is to design new titanosilicate materials based on the acquired knowledge, (theoretically) characterize them from chemical-physical point of view, and provide useful information in order to experimentally synthetize them.
From the practical point of view we have conduced a study on relative stability of mixed titanosilicate nanoclusters with different mixing ratio and with different sizes. During this study we have employed Monte Carlo basin-hopping 4 which is one of the pioneer technique for global optimization problems for the searching of the most stable structure (global minimum). From the results in bulk phase, the mixing of pure titania (rutile) with pure silica (quartz) is thermodynamically unfavorable and this is in agreement with experimental observations which for any mixed system, there is the tendency for titania to segregate in a separate phase from silica. In fact, in crystalline systems, the homogeneous mixing seems to be only possible, by the inclusion of a very small percentage of TiO2 in a silica framework in such a way to avoid contact between TiO2 units. Instead in glassy materials any TiO2:SiO2 mixing ratios is in principle possible but by increasing temperature easily a phase separation occurs. Such bulk phase titanosilicates are metastable (thermodynamically unstable but kinetically stable) with respect to the separate pure polymorphs (quartz and rutile). However, at nanocluster level, from our studies, the mixing of the two phases is thermodynamically favourable, the mixed nanosystems are more stable than the respective pure ones at the same size. Moreover, tracking the relative stability with the size of the clusters we predict that this mixing favourability is persisting up to around 24 unit blocks of SiO2 + TiO2. Furthermore, in our study, we identify some structural factors responsible to the favourability of the mixing, for instance, the average undercoordination of silica which is a destabilizing factor is reduced when titania is present. Although we got very interesting results until now, we haven’t still found experimental collaborators capable to synthetize this nanosystems and to compare their results with our predictions.
1 comment
[…] Algo tan sencillo como mezclar dos óxidos puede tener aplicaciones nanotécnológicas muy diversas. Andi Cuko en Nanoscale mixing: the case of titanosilicates […]