Can a Nitrate Transporter help to reduce the contamination associated to excessive fertilization in agriculture?
Can a Nitrate Transporter help to reduce the contamination associated to excessive fertilization in agriculture?

Nitrogen (N) is an essential nutrient for plant growth and most plants require 20-50 g of N taken up by their roots to produce 1 kg of dry biomass. Although N is one of the most abundant elements on earth, agricultural soils are deficient in N and thus, to sustain crops productivity soils require their supplementation with N compounds. In fact, during the past decades the addition of fertilizers has greatly increased global food production. Indeed, more than 100 million metric tons of nitrogenous fertilizers are added to the soil worldwide annually. However, although necessary for an optimal production, the intensive use of fertilizers has made agriculture one of the major environmental threats. For its production, 1 metric ton of fertilizer synthesized through the Haber-Bosch process requires 873 m3 of natural gas accounting for 1% of global energy consumption every year. Moreover the N release from agriculture systems menaces air, water and soil quality through nitrate (NO3–) leaching into freshwater. Incomplete capture and poor conversion of nitrogen fertilizer also causes global warming through emissions of nitrous oxide (N2O) and reactive N gases (NOx, NH3). One of the most known effects of nitrate leaching is water eutrophication, which can cause algae blooms like the one shown in Figure 1 that leads to oxygen consumption in the water leading even to massive die-off of fish and other animals.
Despite nitrate is dangerous for the environment, it is the most important source of mineral N for plants growing in aerobic soils. Plants uptake NO3– from the soil by absorbing it across the plasma membrane of the root cells. Once inside, NO3– is reduced to ammonium (NH4+) by the action of nitrate and nitrite reductases enzymes and then assimilated into organic molecules like amino acids for plant growth.
We can imagine several ways to reduce nitrate impact in the environment, but all of them rely on the same principle: to apply less nitrate into the agriculture system. This can be achieved among others by the use of fertilizers based on different N forms, like ammonium-based fertilizers, urea or organic fertilizers. Although these represent a promising and sometimes efficient alternative, they present some disadvantages that can lead to crops yield reduction, aspect that will be covered in a future article. Thus, reducing NO3– input into the agriculture system while still using nitrate, inevitably passes through growing plants with higher nitrate use efficiency. Among the different strategies that a plant can deploy to improve its nitrate use efficiency, its uptake is a key aspect and two papers recently published in Nature from a group of the University of Washington, U.S.A. 1 and from the University of Oxford, U.K. 2 shed new relevant light on nitrate transporters regulation.
Nitrate transporters are located in the plasma membrane of root cells. The quantity of nitrate available in the soil fluctuates depending on environmental conditions like temperature influencing microbial activity or water precipitations resulting in nitrate leaching. To cope with this, plants dispose of nitrate transporters with different affinity towards nitrate: NRT1 low affinity and NRT2 high affinity transporters. The first nitrate transporter identified belongs to the NRT1 family and is called NRT1.1. About twenty years ago it was discovered that NRT1.1 is a dual-affinity transporter and about ten years ago it was provided the first evidence on how NRT1.1 could regulate this double affinity unraveling that phosphorylation of a threonine residue (Thr101) was responsible for this change in affinity.
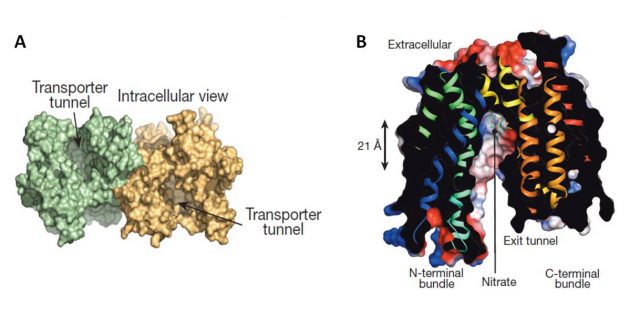
The papers by Sun et al., and Parker and Newstead (2014) provided, by the use of crystallography, new interesting insights on NRT1.1 structure and regulation of its affinity switch. NRT1.1 can be opened towards the inside of the cell, towards the outside, or closed in both sides. Both works resolved about the structure opened facing the cell cytoplasm and identified a histidine residue (His356) as the nitrate-binding site (Figure 2). For instance, the substitution of His356 amino acid by alanine provoked the loss of Arabidopsis thaliana NRT1.1 nitrate binding capacity. Besides, both works showed that NRT1.1 crystallizes as a dimer. However, Parker and Newstead considered dimerization as an artifact of the crystallization procedure and Sun and coauthors proposed a model linking the phosphorylation state of NRT1.1 with its oligomeric state. Sun et al., observed that a mutated NRT1.1 being constitutively phosphorylated on its Thr101 residue provoked the dimer dissociation and proposed that NRT1.1 acts as a high affinity transporter as a monomer (phosphorylated) and as low affinity transporter as a dimer (unphosphorylated). Parker and Newstead proposed that phosphorylation was rather affecting NRT1.1 structural flexibility and associated this with its rate of transport. A smart comment3 about these two articles attempted to bring peace between both models by proposing that the NRT1.1 dimer would have a lower structural flexibility than the monomer (Figure 3).

In summary, contributions towards the molecular understanding of nitrate transport can be essential for future improvement of plants ability to take up nitrate and thus to increase their nitrogen use efficiency. In this sense, resolving NRT1.1 structure provides a valuable basis for future research on nitrate transport which could finally result in the mitigation of some of the detrimental effects of intensive agriculture.
References
- Ji Sun, John R. Banksto2, Jian Payandeh, Thomas R. Hinds, William N. Zagotta and Ning Zheng (2014) Crystal structure of the plant dual-affinity nitrate transporter NRT1.1. Nature 507, 73-77. ↩
- Joanne L. Parker and Simon Newstead (2014) Molecular basis of nitrate uptake by the plant nitrate transporter NRT1.1. Nature 507, 73-77. ↩
- Yi Fang Tsay (2014) How to switch affinity. Nature 507, 44-45. ↩
2 comments
[…] Ingurumen-inpaktu handia duen jarduera da nekazaritza. Inpaktu hau minimizatzeko modu bat da, landareek ongarrietatik hartzen duten nitrogenoaren efizientzia handitzea. Honen inguruan hainbat estrategia daude martxan, euren artean, landareetan nitrogenoa garraiatzen duten mekanismoen erregulazioarena. Daniel Marinok azaltzen digu Can a […]
[…] Una de las actividades humanas con mayor impacto ambiental es la agricultura. Una de las formas de minimizarlo es aumentando la eficiencia con la que las plantas incorporan el nitrógeno de los fertilizantes. Hay varias estrategias en marcha, entre […]