The secret power of lysosomes to cure Parkinson’s and Alzheimer’s
Author:
Pasquale Pellegrini holds an M.Sc. in Biotechnology from Università degli Studi di Bari (Italy). He moved to Barcelona to pursue his Ph.D. at the Bellvitge Biomedical Research Institute (IDIBELL), in Barcelona, where he uncovered key mechanisms of mammary stem cell differentiation and tumor initiation. He is currently a Postdoctoral scholar at the Gladstone Institutes in San Francisco where he is investigating the lysosome network in several neurodegenerative diseases.
What are lysosomes doing inside your cells?
When I was in High School my teacher used to say that lysosomes are only the trash bin of the cell. 20 years later we have discovered a dozen new functions that make this small organelle a core component of the cell.
You may already know that the lysosome pH is extremely low (around 4.5). Such an acidic environment activates lysosomal enzymes that destroy everything including proteins and entire organelles. However, you probably don’t know that lysosomes also regenerate the plasma membrane, regulate the intracellular Calcium and are the control center of energy metabolism 1.
The key function that many laboratories and biopharma companies are trying to elucidate now is how lysosomes regulate autophagy: the process by which every cell degrades its abnormal proteins and entire organelles (see picture below).
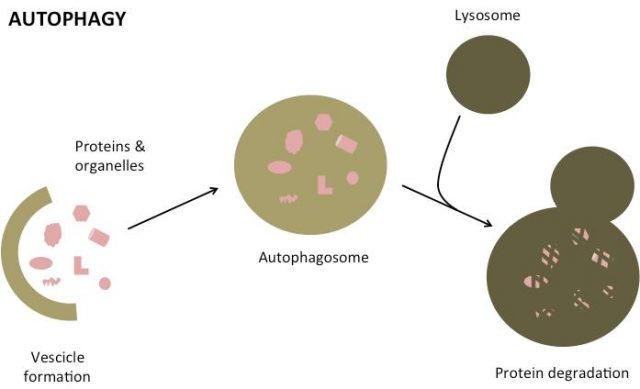
What do lysosomes have to do with Alzheimer’s and Parkinson’s?
Let’s take a step back and think about neuronal life. A neuron does not go through mitosis: if it dies, it’s over,you don’t get a new one. You need your neurons to stay alive and healthy as long as possible, possibly for your entire life. In order to survive for such a long time, neurons use a very efficient cleaning system: the autophagy.
Abnormalities in this process have now been described in several neurodegenerative disorders including Parkinson’s and Alzheimer’s. Dysfunctional autophagy can be due to genetic causes, aging or both.
In familiar cases of Alzheimer’s and Parkinson’s, known to have a strong genetic basis, the genes frequently mutated in these diseases impair lysosome function and autophagy.
An example is the Alzheimer’s-associated gene Presenilin-1, this protein is mostly known to be the main responsible for the formation of neurotoxic Aβ peptide, the main component of the protein aggregates found in brains of Alzheimer patients. A few years ago Dr. Ralph Nixon from the NYU School of Medicine, discovered that this protein, when mutated, impairs lysosome acidification resulting in a lower ability to clear proteins 2.
Similarly, other genes such as Parkin-1 for Parkinson disease and SOD1 for Amyotrophic Lateral Sclerosis when mutated can impair autophagic degradation (Parkin-1) and lysosome movement (SOD1) leading to an abnormal autophagy and accumulation of undigested protein aggregates.
Thanks to genetic studies, the mechanisms underlying protein aggregation is being elucidated. Unfortunately, only 5-15% of the patients present a genetic mutation. The majority of cases manifest as a late-onset sporadic form with no evidence of gene mutations. A full understanding of the causes of the sporadic form is still not yet in reach.
The aged lysosome
Both Alzheimer’s and Parkinson’s disease are age-related progressive neurodegenerative disorders.
Aging has an impact on autophagy: as we get old, this process becomes less efficient 3. Probably the same thing is occurring in the lysosome organelle. In older people, lysosomes accumulate junk that cannot be degraded called lipofuscin. If the autophagy doesn’t work well and lysosomes are not so good to degrade the proteins they receive from the autophagy process we can imagine a scenario where undegraded proteins accumulate and form toxic aggregates that kill neurons.
The promise of fresh new lysosomes
How the cell controls lysosomal function has remained unanswered for a long time. The discovery of a gene network regulating lysosomal biogenesis called CLEAR has been a huge advance in lysosome biology 4.
For the first time, scientists were able to modulate this network to specifically induce the formation of new lysosomes. Apparently inducing the formation of new lysosome was enough to clear protein aggregates and improve cognitive symptoms in mouse models of Alzheimer’s and Parkinson’s disease 5.
However, when we induce the formation of new lysosomes these can fuse with the cell membrane and discharge their content outside of the cell. Such a mechanism can be dangerous in neurodegeneration: what if the secreted toxic proteins are released outside and introduced in another healthy cell? Further studies will be necessary to develop safe lysosome inducers.
An alternative approach to inducing lysosome function comes from the acidic nanoparticles, these particles can enter inside the cell, reach lysosomes and lower the pH. The low pH makes degradation easier because it activates the lysosomal enzymes. The usage of these particles restored the normal pH and reduced the abnormalities in the autophagy pathway in a mouse model of Alzheimer’s disease 6.
A lysosome drug in clinical trials
A promising drug for neurodegenerative diseases is a chemotherapy used for leukemias called Nilotinib. Pre-clinical studies in mouse models of Parkinson’s disease demonstrated that this drug induces the degradation of alpha-synuclein, the main component of protein aggregates, by the lysosomes. Dr. Charbel Moussa from the Georgetown University Medical Center in Washington DC led a trial in 12 volunteers with Parkinson’s disease. The trial was initially designed to test only the safety of the drug via oral administration. Even though the stage of the disease was advanced, all patients started to improve their motility and ability to speak in a few weeks after taking the drug.
“We had people as stiff as a board at the start of the study who were walking around, sitting down and bending their legs by the end,” says Moussa, “You could see the elation on their faces when they saw the improvement. There wasn’t a dry eye in the room.”
The drug was detectable in the cerebrospinal fluid of the participants, demonstrating that it could actually reach the brain of the patients and there was a correlation between the dose of nilotinib and the improvement of the symptoms7. Further clinical trials are planned to confirm the drug’s safety and efficacy.
Final remarks
An estimated seven to 10 million people worldwide are living with Parkinson’s disease. 46 million people worldwide live with dementia, including Alzheimer’s.
There is a growing literature indicating the importance of lysosome function in neurons and neurodegeneration. Developing drugs that induce lysosome function will target the root cause of neurodegeneration and possibly cure these terrible diseases.
References
- Settembre, C., et al., Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat Rev Mol Cell Biol, 2013. 14(5): p. 283-96. doi:10.1038/nrm3565 ↩
- Lee, J.H., et al., Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell, 2010. 141(7): p. 1146-58. doi: 10.1016/j.cell.2010.05.008 ↩
- Rubinsztein, D.C., G. Marino, and G. Kroemer, Autophagy and aging. Cell, 2011. 146(5): p. 682-95. doi: 10.1016/j.cell.2011.07.030 ↩
- Sardiello, M., et al., A gene network regulating lysosomal biogenesis and function. Science, 2009. 325(5939): p. 473-7. doi: 10.1126/science.1174447 ↩
- Ballabio, A., The awesome lysosome. EMBO Mol Med, 2016. 8(2): p. 73-6. doi: 10.15252/emmm.201505966 ↩
- Lee, J.H., et al., Presenilin 1 Maintains Lysosomal Ca(2+) Homeostasis via TRPML1 by Regulating vATPase-Mediated Lysosome Acidification. Cell Rep, 2015. 12(9): p. 1430-44. doi: 10.1016/j.celrep.2015.07.050 ↩
- J. Hamzelou, Drug reversed my Parkinson’s symptoms and let me read again, in New Scientist. 2015. ↩
9 comments
I’ve really appreciate the reading of this article. It is nice to know that there are other way to heal Alzheimer and Parkinson than genetics
Hi Pasquale,
Thanks for such a nice article. Quite enlightening, indeed. First of all I wanted to say congratulations on your postdoc position, I guess Gladstone institutes are quite prestigious research centres. Living in San Francisco must be quite nice, my wife and I fell in love when we went there. Anyway, I was quite shocked about your comments on Nilotinib and Parkinson. I did not know about these clinical (still early, though) results, but in addition…It is a drug already in the market!! I have had a look into this and it´s just amazing. I hope the companies/authorities/research centres involved try to speed up this process, meaning the bureaucracy involved (which is the lengthiest step) to get the permissions is shortened. If efficacy is confirmed I think this will be a huge achievement to the society, as many of us have relatives or know people affected. I actually would like to encourage you to write down a follow-up article getting more focus on this particular aspect. Take care.
Sergio.
[…] The secret power of lysosomes to cure Parkinson’s and Alzheimer’s (mappingignorance.org) […]
Hey Sergio,
Thank you so much! I’m glad you liked it. Yes, Nilotinib looks quite exciting even though I don’t think they know well the mechanism of action. Also this drug was tested only in patients with no controls so I guess it’s still at early stage of the clinical trial. Anyway the research on mouse models is really exciting so hopefully we will have new drugs in the near future.
Cheers
Pasquale
Thank you for the article. I really enjoyed reading about lysosomes and their secret power to cure dementias. Advances in lysosomes may allow us to stop the neurodegenerative pathology.
Now, re: Alzheimer’s disease, does stopping pathology will really mean stopping behavioural damage? How about other brain pathology that lysosomes will probably not cure, such as vascular damage or periventricular white matter damage? or even other behavioural factors that may appear without a clear brain pathology?
Thank you again!
Hey!
It’s not known if these therapies can cure behavioural deficits or other forms of dementia caused by vascular damage. There are some data in mouse models where they show some rescue of behavioural damage but in humans it’s a completely different story!
I think that the abnormalities of the autophagy-lysosome system are very early events in the disease. Formation of protein aggregates and release in the extracellular matrix is most likely a consequence of these abnormalities.
My understanding is that drugs that showed to be ineffective in patients target these extracellular aggregates (like beta amyloid). These extracellular aggregates correlate with the disease but are not the driving cause that kill neurons. Many people hypothesize that the intracellular oligomers and other aggregates are the most neurotoxic. The lysosome can’t bear all this amount of junk so they accumulate and dysregulate multiple pathways inside the cell leading to cell death.
That’s why lysosome inducers look so appealing. 🙂
[…] The secret power of lysosomes to cure Parkinson’s and Alzheimer’s […]
Hi Pasquale,
Thanks for such an informative article.
I have GBA mutation resulting in Gaucher’s disease and within the past year I have been suffering from Gaucher induced Parkinsonism. As the enzyme replace therapy that I receive for Gaucher doesn’t cross the blood brain barrier, I am hopeful that drugs like Nilotinib will be beneficial.
Regards,
Michael
how can we help fund this research?