Beryllium: closer than ever, not bonded though

What is the closest two beryllium atoms in the electronic ground state can be in a molecule? Well, we may think that that is a relatively easy question to answer. If you know some chemistry it is likely you will be inclined to check the literature for the strength of Be-Be bonds in different compounds, find the higher one, and then check de distance in that structure; and there you have the answer. Well, incredibly as it may seem, that is not the case.
Beryllium forms dimers in some compounds, and we could think that in some of them the bond would be strong and the distance quite short. Actually de Be-Be dimer can form with a triplet state of Be and a distance of just 1,811 Å. However, calculations show that in the electronic ground state of neutral Be2 the distance grows to 2.534 Å.
We talk about calculations because none of the diatomic species with a strong Be-Be bond, that is, Be2 in the electronic state, nor the cations Be2+ and Be22+ have been observed experimentally. The known toxicity of beryllium may be the reason for the lack of experiments. In any case, is it possible to induce a strong Be-Be bond in the electronic ground state of a neutral molecule?
The system that set the record for the shortest and strongest Be-Be bond in neutral systems calculated thus far is (NHCH)Be-Be(NHCH) at 1.945 Å, where NHCH represents a N-heterocyclic carbene where hydrogen is attached to the N atoms.
Now a team of researchers from Jilin University, Marburg University and professor Gernot Frenking from DIPC, has extended the search for the shortest distance between beryllium atoms in the ground electronic state in a neutral molecule. With surprising results that are published in Angewandte Chemie 1.
In the process they found interesting things. For example that in Be2F2 the distance is not shorter than in the NHC complex (2.048 Å versus 1.945 Å) but, remarkably, its bond dissociation energy is larger,76.9 kcal/mol versus 39.8 kcal/mol, setting a new record for a Be-Be bond.
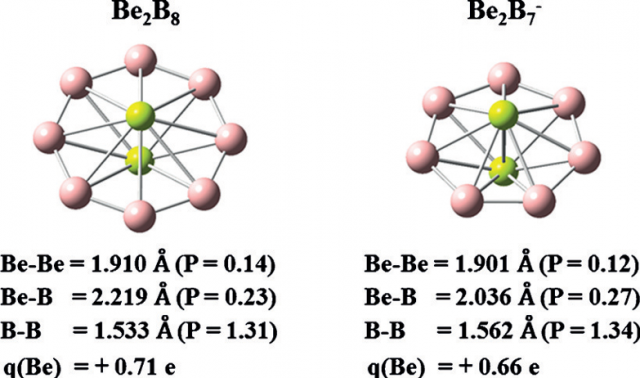
But the real deal are two molecules where the Be–Be axis is perpendicular to rings of boron atoms (Figure 1), Be2B8 and Be2B7–, which exhibit a discus-shaped equilibrium geometry. The calculations suggest that Be2B8 and Be2B7– are doubly aromatic molecules with a 6s and 6p electron structure. There are eight (seven) further classical σ molecular orbitals in Be2B8 (Be2B7–), which describe the B-B σ bonds. But, and this is a big but, there are no direct occupied Be-Be bonding orbitals as there is only indirect attraction between the beryllium atoms via the aromatic system.
The result is that B-B bonds in these cyclic species are 1.533 Å in Be2B8 and 1.562 Å in Be2B7– . Most importantly, the interatomic distance between the beryllium atoms are significantly shorter than any other known: 1.910 Å and 1.901 Å, respectively . This is due to the Be-B σ and π bonds, which operate like spokes in a wheel pressing the beryllium atoms together. It is the formation of the Be-B bonds what has effectively removed the electronic charge of the valence space between the beryllium atoms.
In other words, two beryllium atoms in the electronic ground state are the closest in a molecule where there is no bond between them. Go figure.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
References
- Zhong-hua Cui, Wen-sheng Yang, Lili Zhao, Yi-hong Ding, and Gernot Frenking (2016) Unusually Short Be-Be Distances with and without a Bond in Be2F2 and in the Molecular Discuses Be2B8 and Be2B7– Angewandte Chemie doi: 10.1002/anie.201601890 ↩