Novel methods of chiral separation
Author: Adrián Matencio is pursuing a PhD at the Department of Biochemistry and Molecular Biology – A, Faculty of Biology, University of Murcia, where he graduated in biotechnology.
A long time ago, Aaron Burr Jr. (1756 –1836), the Vice-President of the United States under Thomas Jefferson said something that is still applicable today: “The rule of my life is to make business a pleasure, and pleasure my business”. This is because current society demands the same things as Burr society: good food, free time with family and friends, and the more healthy you are and longer you live, the better.
The industry also knows that these things are necessary, which is why they have developed a huge range of products, increasing interest in finding new ingredients with high added value, for example. In recent years, plants have been studied to find new products, and furthermore, many researchers have focused on improving the production of bioactive secondary metabolites in plants. One way to do this is to increase the yield using a technique called elicitation, which involves using certain molecules or conditions to enhance the production of a given bioactive compound in plant cell cultures 1. In this case, we shall focus on one molecule, Methyl Jasmonate (MeJA, Fig. 1); (this molecule is involved in the production of some bioactive compounds both in plants and humans, for example, resveratrol 2, Taxol® etc.
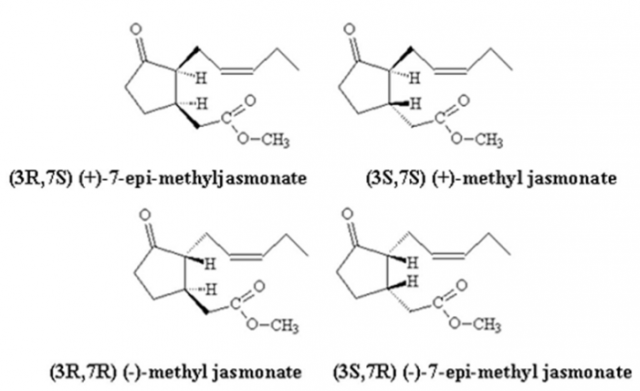
Nevertheless, the commercial MeJA is a mixture of the four stereoisomers (Fig. 1): (+)epiMeJA, (-)epiMeJA, (+)MeJA and (-)MeJA and when the effect of post-harvest treatment with stereoisomers of MeJA on the bioformation of myricetin, quercetin and kaempferol was studied in red raspberry, the results obtained depended on the stereoisomer used 3. This is important because, if we used one of the above, we would obtain a higher concentration of the bioactive compounds of interest without mixing it with other isomers. Several methods have been created to obtain the four stereoisomers: asymmetric synthesis, enzymatic resolution and HPLC resolution prior to diastereoisomer formation 4.
However, these methods are expensive and difficult to carry out so our research group 5 successfully separated and identified all four stereoisomers using a simple and sensitive HPLC-RP method. To do this, we used cyclodextrins (CDs, doughnut-shaped oligosaccharides made up of α-[1-4]-linked glucose units) in the mobile phase of the HPLC (high-performance liquid chromatography). CDs can not only complex hydrophobic compounds to form inclusion complexes (Fig. 2), but can separate isomer compounds using their strong affinity for each other. HPLC can be thought of a 400 meters hurdles race, in which the best-trained athlete will win the race. It is a very useful technique for separating compounds and, if we introduce CDs too at the same time as the MeJA mixture, the four stereoisomers will be better separated.
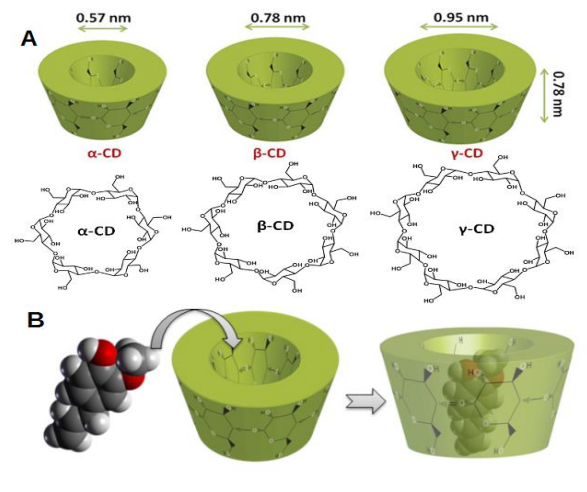
Bearing the above in mind, our first step was to test the efficiency of many types of CDs to encapsulate MeJA 6. Of all the CDs tested, Methyl-beta-cyclodextrin (MβCD) was the best for encapsulating MeJA. Its complex constant (KF, a value to express the strength of the encapsulation) was almost double that of the second best CD (Table 1). Moreover, the isomers were poorly separated by only using MβCD.
Table 1. Apparent KF values and correlation coefficients for different MeJA-CD complexes at 25ºC in a MeOH/water (40:60%) medium compared with our results for the MeJA/MβCD complex.
| Correlation coefficient | |||
| Complex | KF (M-1) | 1:1 | 2:1 |
| MeJA/αCD | 30.4 +/- 1.5 | 0.99 | 0.91 |
| MeJA/βCD | 60.9 +/- 2.3 | 0.99 | 0.86 |
| MeJA/γCD | 9.6 +/- 0.08 | 0.99 | 0.92 |
| MeJA/HPβCD | 43.1 +/- 1.6 | 0.99 | 0.89 |
| MeJA/HEβCD | 22.8 +/- 1.1 | 0.99 | 0.89 |
| MeJA/MβCD | 117.49 +/- 5.9* | 0.99 | 0.87 |
* Major peak used
Based on these results, we tried to obtain a better separation the four stereoisomers. The following conditions of our experiment were changed: the length of the column, temperature of process, percentage of organic solvent and quantity of MβCD. Finally, we obtained 4 peaks, one per stereoisomer (Fig. 3) using a longer C18 column (250 mm), a lower organic solvent percentage (20% of methanol), a higher MβCD concentration (16 mM) and a lower flow rate (1.25 mL/min).
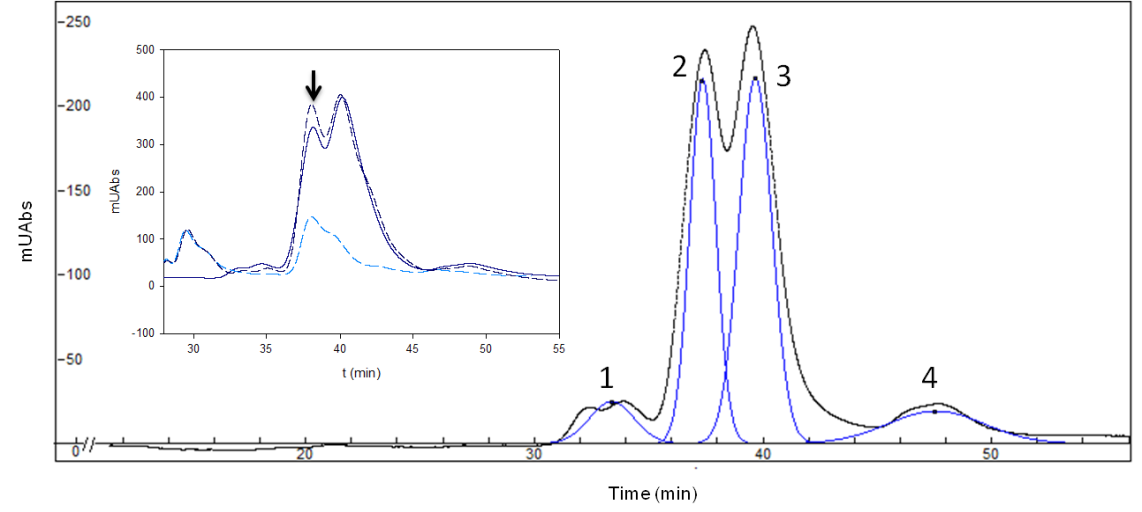
However, the most difficult part of this work was had still not been solved – which peak represents each isomer? We thought of two different strategies, 1) an in silico docking simulation, which obtained the following peak order: Peak 1: (-)EpiMeJA: Peak 2: (-)MeJA; Peak 3: (+)MeJA and Peak 4: (+)EpiMeJA. Even so, since no isomer is available commercially, we used a commercial sample of Rosemary essential oil, pure sample of (-)MeJA 7 to coinject with our mixture in HPLC. If our second peak, which was predicted to be (-)MeJA, really was (-)MeJA, it would increase due to the rosemary essential oil. And, perhaps surprisingly, the peak increased, confirming that the second peak was (-)MeJA (Fig. 3 insert).
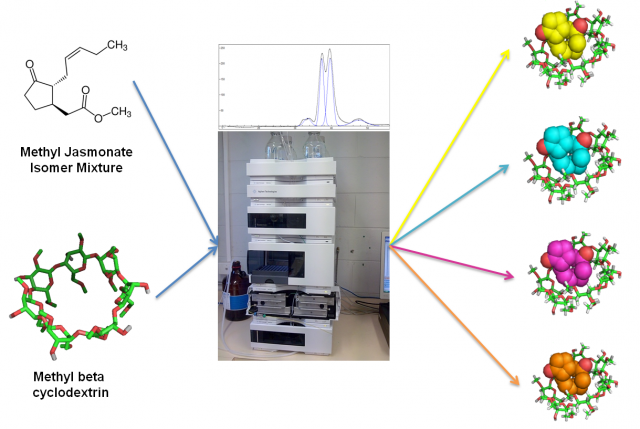
To sum up, in this article we have seen how MβCD can be used as additive in a simple HPLC-RP setup to separate the four stereoisomers of MeJA. In this case, a combination of in silico and co-injection techniques was used to identify the order of the peaks. These results (Fig. 4) could be used to improve the purification of each isomer and to obtain new bioactive compounds for the nutraceutical, cosmetic and pharmaceutical industries.
References
- Karuppusamy, S. (2009). A review of trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. Journal of Medicinal Plants Research, 3(13), 1222-1239. ↩
- Almagro, L., Belchí-Navarro, S., Sabater-Jara, A. B., Vera-Urbina, J. C., Sellés-Marchart, S., Bru, R., & Pedreño, M. A. (2013). Bioproduction of trans-resveratrol from grapevine cell cultures. In Natural Products (pp. 1683-1713). Springer Berlin Heidelberg. ↩
- de la Peña Moreno, F., Blanch, G. P., & Ruiz del Castillo, M. L. (2010). (+)-Methyl jasmonate-induced bioformation of myricetin, quercetin and kaempferol in red raspberries. Journal of agricultural and food chemistry, 58(22), 11639-11644. ↩
- Flores, G., Blanch, G. P., & del Castillo, M. L. R. (2013). Isolation of the four methyl jasmonate stereoisomers and their effects on selected chiral volatile compounds in red raspberries. Food chemistry, 141(3), 2982-2987. ↩
- Matencio, A., Bermejo‐Gimeno, M. J., García‐Carmona, F., & López‐Nicolás, J. M. (2017). Separating and Identifying the Four Stereoisomers of Methyl Jasmonate by RP‐HPLC and using Cyclodextrins in a Novel Way. Phytochemical Analysis, 28(3), 151-158. doi: ↩
- López-Nicolás, J. M., Escorial Camps, M., Pérez-Sánchez, H., & García-Carmona, F. (2013). Physicochemical and thermodynamic characterization of the encapsulation of methyl jasmonate by natural and modified cyclodextrins using reversed-phase high-pressure liquid chromatography. Journal of agricultural and food chemistry, 61(47), 11347-11354. ↩
- Ruiz del Castillo, M. L., & Blanch, G. P. (2007). Enantiomeric purity of (+/–)‐methyl jasmonate in fresh leaf samples and commercial fragrances. Journal of separation science, 30(13), 2117-2122. ↩
1 comment
[…] Lo que los químicos llaman (llamamos) resolución de racematos en estreoisómesos, esto es, simplificando, ser capaz de separar dos moléculas que son la imagen especular la una de la otra, no es tarea sencilla pero sí tiene un enorme […]