Superparamagnetic nanoparticles and the separation problem
It has been a long time since we have learned that “going nano” leads to new properties arising from matter. One of those size-dependent properties that promises huge benefits due to its potential applications is magnetism. Magnetic materials are classified according to their susceptibility to magnetic fields into diamagnetic, paramagnetic and ferromagnetic materials, the first two categories behaving in a way that their magnetic properties don’t persist when the external magnetic field is removed. On the contrary, in ferromagnetic materials their magnetic properties continue even when the external magnetic field is removed. These materials are usually referred to as magnets. When magnets are subjected to the effect of a magnetic field the individual magnetic moments of their atoms align with this field, and when the field is removed part of this alignment is retained: the material has been magnetized.
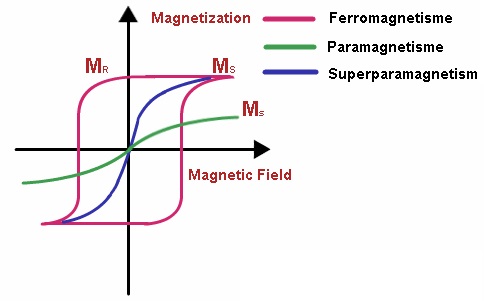
Within a magnet we find different regions or domains differing each of them in the direction of the magnetic moments’ alignment. The reason for this separation is energy-related: the higher the number of domains, the less the internal energy of the material. As the size of the material is reduced we get closer to the limit in which an only single domain is observed. These single-domain magnets align all their magnetic moments in the same direction, so that when an external magnetic field is applied the resulting magnetization is the largest possible for that specific material and size. Here comes the superparamagnetic behaviour, which shares with paramagnetism the absence of magnetization when the external magnetic field is removed and with ferromagnetism the high levels of magnetization reached under the influence of a low magnetic field.
Superparamagnetism appears, depending on the materials, in particles below 50 nm with a single magnetic domain. Besides the above mentioned, other interesting properties of superparamagnetic nanoparticles are their short relaxation time, that is, the time in which magnetization goes to zero after the effect of an external magnetic field (typical values are around 10-9-10-10s), and the fact that under the effect of an alternating external magnetic field, their magnetic moments are quickly reoriented so that changes in the frequency and strength of the magnetic field lead to a loss power that heats the surrounding environment.

The unique properties of superparamagnetic nanoparticles have found numerous applications in the biomedical field, such as tissue repair by local heating, detoxification of biological fluids, magnetically controlled delivery of drugs or genes, or as contrast agents in magnetic resonance imaging (MRI). All of them are being thoroughly investigated and there are still many challenges to deal with, mostly related to safety issues. The consequences of the introduction of nanoparticles inside the body are not yet fully understood, and there are grounds for thinking that their interaction with living systems can cause long term toxicological effects that cannot be dismissed, as it was exposed in a previous article.
Figure 3. Superparamagnetic nanoparticles can act as cell labeling agents for magnetic resonance imaging (MRI) due to their high magnetic susceptibility. | Credit: In vivo Molecular Imaging Research group (IMIR) KU Leuven
Another interesting application in which superparamagnetic nanoparticles could be decisive is the magnetic removal of hazardous substances from water, substituting complex filter systems with even more complex maintenance. According to environmentalists, water pollution is a global problem of utmost importance and the major worldwide cause of deaths and diseases. Whether due to human activity or naturally occurring, the water contamination problem must be faced by the development of new and more efficient technologies. The role of superparamagnetic nanoparticles in water-cleaning is the adsorption of harmful substances to the nanoparticles’ surface followed by their subsequent removal by means of a low magnetic field gradient.
It seems that the adsorption of contaminants to the nanoparticle surface is the easy part of the process. The physico-chemical affinity is achieved via functionalization of the nanoparticle surface in order to the specific contaminant to get attached to it. The great advantage of going nano in that the high surface/volume ratio enhances the scavenger process efficiency several orders of magnitude. However, the real challenge seems to be the separation of the magnetic nanoparticles from water once the adsorption has taken place, what has been called the separation problem.
Dr. Frank Hutter from the Fraunhofer Institute for Silicate Research (ISC) in Germany has studied magnetically separable nanoparticles exhaustively and has recently published several articles addressing important advances in this field1. He points out that if we want to take advantage of the nano-size effect, a proper dispersion of the magnetic nanoparticles must be achieved, otherwise agglomeration will lead to a poor adsorption of water contaminants, even if the subsequent magnetic separation can be easily carried out. However, a good nano-dispersed solution entails other kind of problems. First of all, in aqueous systems nanoparticles stabilization is achieved via electrostatic repulsion between the particles, which can be problematic in real water due to the existence of ions preventing this electrostatic effect. Secondly, it has been observed that nano-dispersions present Brownian forces several orders of magnitude larger than the magnetic forces applied for their separation in aqueous systems, so it seems that a balance must be found between a fully nano-dispersed solution and certain degree of agglomeration, enough for the magnetic field to beat the Brownian forces originated.

In any case, further understanding of nanoparticles’ behaviour in fluids and magnetic fields is needed in order to make the most of this potential technology. This includes the use of ferrofluids, a colloidal suspension of magnetic nanoparticles in a continuous liquid medium. Water miscible ferrofluids could act as a comb to clean water and then be attracted by a magnet as it is shown in the picture below.

The potential applications of these systems are yet to be fully discovered. Most of them are focus on the removal of heavy metals from water; Dr. Hutter has studied the elimination of mercury and copper, and also the naturally occurring arsenic contamination of groundwater. Other research has been focused on the removal of organic contaminants, such as crude oil spills. Researchers from the Texas A&M University have published an interesting work2 in which an hybrid nanomaterial consisting of amphiphilic block copolymers and oleic acid-stabilized magnetic iron oxide nanoparticles successfully act trapping crude oil spills in water. They claim that the oil-loaded magnetic nanoparticles are easily removed under the effect of a low magnetic field. Moreover, after their recovery, the nanoparticles can be recycled and re-employed enhancing the effectiveness of the whole process.
To sum up, superparamagnetic nanoparticles seem to have a very promising future not only in the biomedical field, but also as a powerful environmental technology. Nevertheless, there is still an open question about the potential toxicity of these nanomaterials that cannot be ignored. In the meantime we will be attentive to new advances in this field.

References
- K. Mandel, F. Hutter, The magnetic nanoparticle separation problem, Nano Today (2012) 7, 485-487. http://dx.doi.org/10.1016/j.nantod.2012.05.001 ↩
- K. Mandel et al, Modified Superparamagnetic Nanocomposite Microparticles for Highly Selective HgII and CuII Separation and Recovery from Aqueous Solutions, ACS Applied Materials and Interfaces, (2012) 4, 5633-5642. http://dx.doi.org/10.1021/am301910m. ↩
4 comments
[…] Nanomaterialetan jartzen dugun itxaropena, eskala nanometrikoan azaltzen diren propietateetan oinarritzen da batez ere. Biomedikuntzan aplikazio ugari dituen superparamagnetismoa da aipatutako propietatetariko bat. Hala ere, gorputzean nanopartikulak txertatzearen ondorioak ez dira oso ezagunak. Superparamagnetismoaren beste aplikazio posible bat ur-arazketa da […]
[…] Muchas de las esperanzas puestas en los nanomateriales se basan en propiedades que sólo aparecen a escala nanométrica. Una de ellas es el superparamagnetismo que ha encontrado múltiples aplicaciones en biomedicina, auqnue los efectos de introducir nanopartículas en el […]
Dear Dr Silvia Roman
Greeting
I am teacher with biological background and teaching nanotoxicology . Your explanation on super para magnetic topic is very lucid and a complex topic is expressed in simple way.
Keep it up GOOD JOB
Dr Y K Lahir, Dept of Biophysics, University of Mumbai, Mumbai, India
Dear Silvia Román
It was great. Informative and simple