When we forget basic science. An example concerning solubility
Author: Adrián Matencio is pursuing a PhD at the Department of Biochemistry and Molecular Biology – A, Faculty of Biology, University of Murcia, where he graduated in biotechnology.
Nowadays, most important news concerning science focuses on applicability; if you read a newspaper or magazine you will see news about how a new drug or appliance can be applied in the fight against disease, hunger, etc. However, behind the results lies a lot of hard work on the part of different group s- perhaps in many different countries throughout the world – who may, for example, have been studying the background of a molecule for man years. Meanwhile, and important characteristic such as its stability or solubility may have been overlooked if a study focuses on applicability.
In the present article, we shall talk about an example that my research group encountered in the case of α-transMethylStilbene (tMS), a compound with a similar structure to Resveratrol, well known for its many bioactive properties as an antioxidant, anti-inflammatory, anticarcinogenic, etc. 1..
One of the strategies when seeking bioactive compounds is to test analogues – drugs with similar structure – of an active compound. For this reason, tMS was tested in anticancer high throughput screenings, a type of experiment that uses machines and automatic processes to rapidly research millions of chemical, genetic, or pharmacological tests. However, the structure of tMS Fig. 1) reflects its very low solubility, which is almost 0 in water, despite which it was administrated in a saline solution of water in these experiments. Although it was inactive in the test, it is clear that a compound that is insoluble in water should not be administered in saline solutions before optimizing its solubility. Indeed, omitting this step could prevent its possible bioactivities from being discovered.
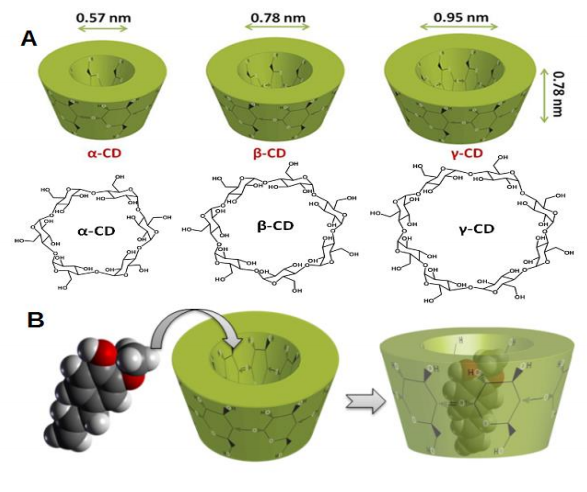
For this reason, our research group studied how its solubility might be increased by complexation with cyclodextrins (Fig. 2A, donut-shaped oligosaccharides made up of α-[1-4]-linked glucose units) in water 2. Cyclodextrins (CDs) may not only complex hydrophobic compounds to form inclusion complexes (Fig. 2B), but the complex itself may increase the apparent solubility of the drug creating a reservoir or drug that can be released. Moreover, natural CDs can be used as food additive and in the pharmaceutical industry 3.
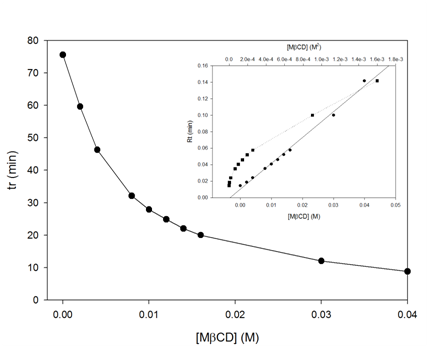
The first step of our investigation was to study the possible complexation of tMS with CDs. To do this, we added different concentrations of CDs in the mobile phase of a HPLC (high performance liquid chromatography) apparatus. HPLC can be thought of a 400 meter hurdles race, in which the best trained athlete will win. So, if we introduce CDs in the mobile phase at the same time as tMS, it will become more hydrophilic as a result of its complexation and its retention time will decrease. Plotting the retention time (TR) against increasing concentration of each CD (Fig. 3) provided an exponential decrease curve. This indicates that the use of CDs would create a CD/tMS complex, which is more hydrophilic, increasing tMS solubility.
Different natural and modified CDs were used and an apparent complexation constant (KFapp) for each one was obtained using an appropriate mathematical (see Matencio et al, 2017) expression (Table 1). Furthermore, each CD formed a 1:1 complex (1 CD per tMS). This is important, for example, for optimizing the quantity of CD to be used with the tMS. The results indicated that the semi-synthetic Methyl-βCD (MβCD) would be the best CD to increase the concentration of tMS in aqueous solutions.
Type de CD | KFapp (M-1) | SD (+/-) | R2 (1:1) | R2 (1:2) |
| MβCD | 298.01 | 15.03 | 0.99 | 0.95 |
| HPβCD | 108.65 | 6.02 | 0.99 | 0.95 |
| βCD | 82.67 | 4.01 | 0.99 | 0.95 |
| γCD | 23.16 | 1.12 | 0.99 | 0.96 |
| αCD | 12.15 | 0.80 | 0.99 | 0.95 |
Table 1. Experimental KFapp values and correlation coefficients for 1:1 and 1:2 tMS/CD complexes (the higher, the better) respectively, at 25 ºC in C18 column and 60:40 MeOH:water.
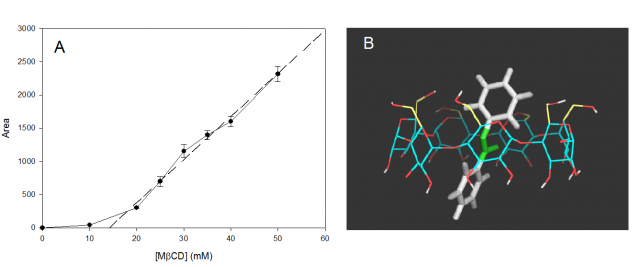
However, this observation needed to be explained and so the following experiment was prepared to increase the solubility of the compound. Different water solutions were prepared using the same quantity of tMS (in oversaturated conditions) and increasing concentrations of MβCD and left for 24 hours (during this time, the complex would be formed and the apparent solubility of tMS should increase). Then, we removed the non-soluble part of tMS and measured the liquid by HPLC. Fig. 4A shows the results of this experiment, where it is very clear that the concentration of tMS increases when the concentration of MβCD increases. It can also be seen how the solubility of tMS without CDs is almost zero. The conclusion was that without CDs or any similar complexing agent, tMS is not soluble in water or saline solutions.
On the other hand, how does tMS interact with CDs? To understand this, a molecular docking simulation was carried out (Fig. 4B), the result showing that only hydrophobic interactions take part in the complex stabilization. The structure of tMS was seen to have no hydrophilic group to form hydrogen bonds.
To sum up, we have seen how MβCD can be used as additive to increase the apparent solubility of a tMS through the formation of a inclusion complex that creates a reservoir of available tMS. In this case, direct and indirect measurement of the solubility was carried out with the same results: only by adding CD can any quantity of tMS be dissolved in water or saline solutions. These study shows why basic science is necessary for developing science; by not taking into account such a simple thing as solubility, the results would not have reflected what is possible.
References
- Pangeni, R., Sahni, J. K., Ali, J., Sharma, S., & Baboota, S. (2014). Resveratrol: review on therapeutic potential and recent advances in drug delivery. Expert opinion on drug delivery, 11(8), 1285-1298. ↩
- Matencio, A., Hernández-García, S., García-Carmona, F., & López-Nicolás, J. M. (2017). An integral study of cyclodextrins as solubility enhancers of α-methylstilbene, a resveratrol analogue. Food & Function, 8(1), 270-277. doi: 10.1039/C6FO01677D ↩
- Del Valle, E. M. (2004). Cyclodextrins and their uses: a review. Process biochemistry, 39(9), 1033-1046. ↩