When we forget basic science. A jar is not a jug
Author: Adrián Matencio is pursuing a PhD at the Department of Biochemistry and Molecular Biology – A, Faculty of Biology, University of Murcia, where he graduated in biotechnology.

Applicability or some usefulness is the mantra of new I+D research. However, in trying to obtain results or confirm an application we often forget to check or study the conditions and methods used. One example could be the different methodologies that we can be used to define antioxidant capacity. An antioxidant is a molecule that inhibits the oxidation of other molecules. To balance the oxidative state, plants and animals maintain complex systems of overlapping antioxidants, such as glutathione and enzymes (e.g., catalase and superoxide dismutase), which are produced internally, ingesting dietary antioxidants (vitamin A, vitamin C, and vitamin E) or bioactive compounds (flavonoids, caffeine, carotenoids, etc).
While a given method for measuring antioxidant capacity may be applied several times, different results might be obtained depending on the temperature, pH, bioactive compound, etc.
In the present article, we shall look at an example that my research group encountered when working with stilbenes, a group of compounds that includes Resveratrol, which is well known for its many bioactive properties as an antioxidant, anti-inflammatory, anticarcinogenic, etc. 1.
Several techniques can be used to measure the antioxidant capacity, such as ORAC; FRAP or ABTS+. But since each technique focuses on a different principle, why should I expect to have the same result.
We selected four stilbenes (Figure 1), with a different number of hydroxyl groups (a well-known antioxidant group in organic molecules). It might be expected that the more hydroxyl groups, the better the antioxidant capacity. However, other factors could affect the power of each group, such as the chemical environment.
Our first step was to test 2 their antioxidant capacity using the three different techniques: ORAC; FRAP or ABTS+ (ORAC is a hydrogen atom transfer method while others are single electron transfer methods). The results (Table 1) demonstrated how each technique provides a different ordination and TEAC value (Trolox equivalent antioxidant capacity).
| Molecule by Number of –OH’s | Group 1 | Group 2 | |
| ORAC | ABTS+ | FRAP | |
| Oxyresveratrol | 3.35 +/- 0.2 | 2.04 +/- 0.12 ↑ | 0.52 +/- 0.1 ↑ |
| Resveratrol | 3.67 +/- 0.2 | 1.57 +/- 0.1 ↓ | 0.47 +/- 0.1 ↓ |
| Pinosylvin | 1.71 +/- 0.1 | 1.23 +/- 0.1 ↑ | 0.31 +/- 0.1 ↑ |
| Pterostilbene | 2.57 +/- 0.1 | 1.13 +/- 0.1 ↓ | 0.05 +/- 0.1 ↓ |
Table 1. TEAC values (in µM) for each stilbene and technique (arrows indicate the relative change of ordination according to the ORAC experiment).
It is clear now that not all the techniques give the same results. Indeed, the ORAC assay gives an ordination unrelated with the number of hydroxyl groups. In this case, several different factors may have intervened: the technique itself or the chemical environment, intramolecular resonance, among others… On the other hand, group two (ABTS+ and FRAP) gives the same ordination but a different absolute value of TEAC, (although the principle is the same, different reagents are used in each technique).
Furthermore, we tested the influence of pH on antioxidant capacity. Stilbenes are a family of hydrophobic compounds and some authors have worked at values as high as pH 8 or 8.5, where their solubility is higher 3. Besides the fact that this pH is not representative of human values (around 7.4 or less), increases the concentration of stilbenes, above their real degree of solubility in cells.
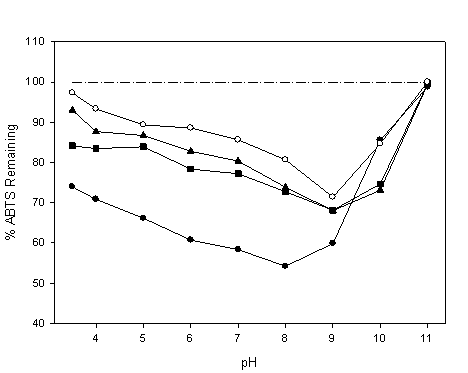
Our results (Figure 2) demonstrated that the pH strongly affects the antioxidant capacity. The higher the pH, the greater antioxidant capacity, with a maximum at pH 9 (8 for oxyresveratrol). However, for the study to be representative of what occurs in human tissues, a lower pH must be used. To increase the antioxidant capacity of stilbenoids at a low pH, we could try to increase the quantity of bioactive compound at that pH using different strategies such as encapsulation 4.
To sum up, we have seen how different techniques (or the same technique used in different conditions) do not always give the same ordination of antioxidant capacity and the results will depend on pH, molecule, temperature, etc. This study shows again why basic science is necessary for developing science; if we do not choose the most suitable technique for our study, the results may not reflect what is really happening.
Editor’s note: The headline is a short version of an old Spanish adage, ‘Aunque todo sea barro, no es lo mismo tinaja que jarro’, that could be translated as ‘Even though both are made of clay, a jar is not a jug’.
References
- Pangeni, R., Sahni, J. K., Ali, J., Sharma, S., & Baboota, S. (2014). Resveratrol: review on therapeutic potential and recent advances in drug delivery. Expert opinion on drug delivery, 11(8), 1285-1298. ↩
- Rodríguez-Bonilla, P., Gandía-Herrero, F., Matencio, A., García-Carmona, F., & López-Nicolás, J. M. (2017). Comparative Study of the Antioxidant Capacity of Four Stilbenes Using ORAC, ABTS+, and FRAP Techniques. Food Analytical Methods, 10(9), 2994-3000. doi: 10.1007/s12161-017-0871-9 ↩
- López-Nicolás, J. M., & García-Carmona, F. (2008). Aggregation state and p K a values of (E)-resveratrol as determined by fluorescence spectroscopy and UV− visible absorption. Journal of agricultural and food chemistry, 56(17), 7600-7605. ↩
- López-Nicolás, J. M., Rodríguez-Bonilla, P., & García-Carmona, F. (2014). Cyclodextrins and antioxidants. Critical reviews in food science and nutrition, 54(2), 251-276. ↩
1 comment
[…] Antioxidatzailea da hori? Zelan neurtzen duzun arabera. Estilbenoen kasua Adrián Matencioren eskutik When we forget basic science. A jar is not a jug […]