The 7 states of condensed matter at room temperature
In ‘Chemistry Essentials for Dummies‘, John T. Moore writes:
Matter is anything that has mass and occupies space. It can exist in one of three classic states: solid, liquid, and gas. When a substance goes from one state of matter to another, the process is called a change of state, or phase change.
As he puts it, this a classical and popular view of states of matter where there are only two states of condensed matter, namely, solids and liquids. But this differentiation is not useful from the moment we are interested for technical or scientific reasons in them. Some more precision when it comes to differentiate states of condensed matter is needed. If we should mention those that we may find in our daily life outside a laboratory and, to narrow the search a little bit, are stable at room temperature (some 20 ºC), we need to focus on how atoms or molecules relate to each other. We’ll find that the two classic ones turn into, at least, seven.
Atomic crystals
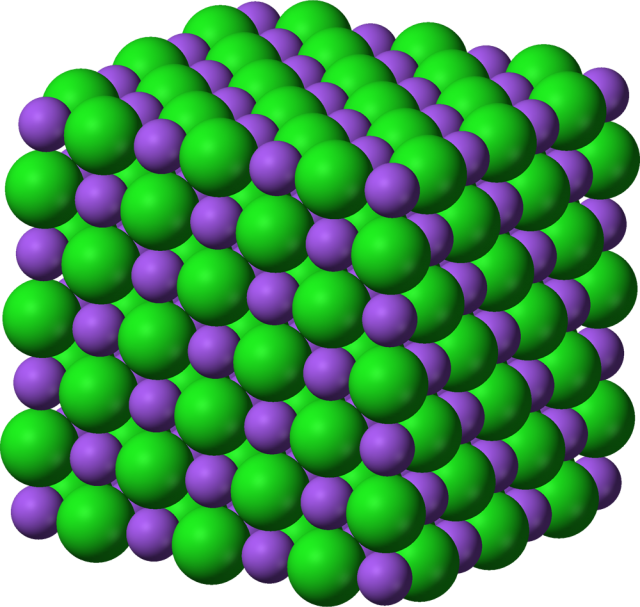
Atoms can bond together the form regular crystals. A crystal is made of small units reproduced many times and built into a regular array.
Molecular crystals

It is also possible that atoms will bind together to form molecules, and the molecules will stick together via weak Van der Waals bonds to form so-called molecular crystals.
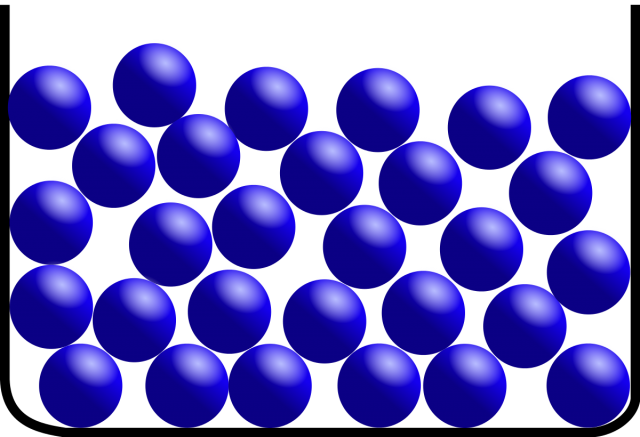
Liquids

Here, atoms are attracted to each other, but not so strongly that they form permanent bonds (or the temperature is high enough to make the bonds unstable). Liquids (and gases) are disordered configurations of molecules where the molecules are free to move around into new configurations.
Amorphous solids and glasses
Somewhere midway between the idea of a crystal and the idea of a liquid is the possibility of amorphous solids and glasses. In this case the atoms are bonded into position in a disordered configuration. Unlike a liquid, the atoms cannot flow freely.
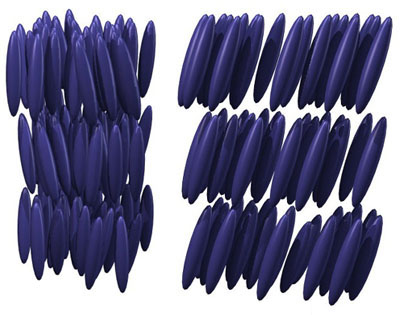
Liquid crystals

The system orders in some ways but remains disordered in other ways. For example, in so-called smectic phases the system is crystalline (ordered) in one direction, but remains disordered within each plane. One can also consider cases where the molecules are always oriented the same way but are at completely random positions (known as a “nematic”). There are a huge variety of possible liquid crystal phases of matter. In every case it is the interactions between the molecules (“bonding” of some type, whether it be weak or strong) that dictates the configurations.
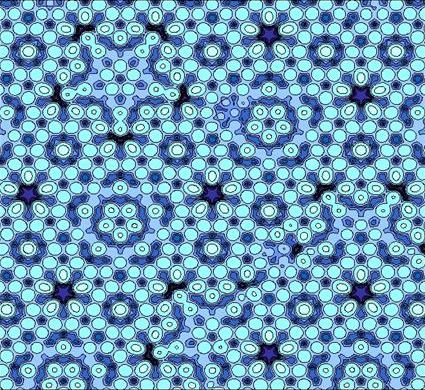
Quasicrystals
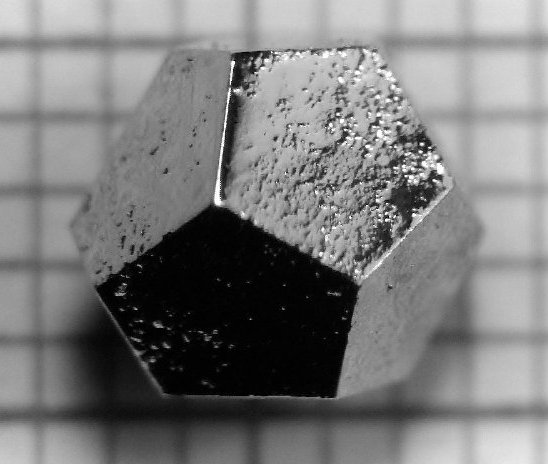
These are ordered but nonperiodic arrangements. In a quasicystal component units are assembled together with a set of regular rules which appears to make a periodic structure, but in fact the pattern is non-repeating.
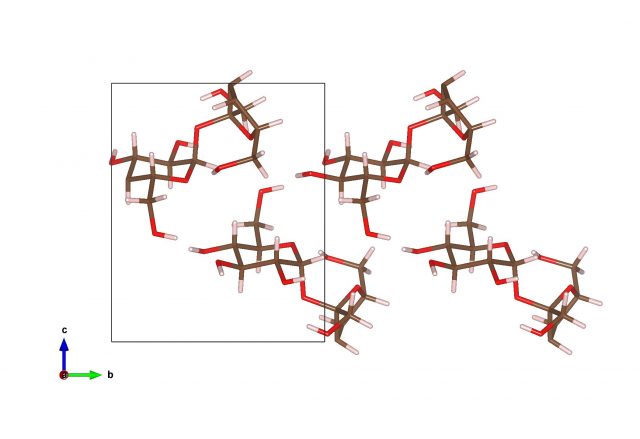
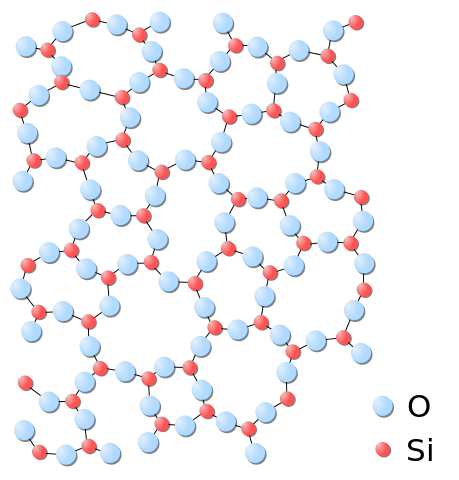
Polymers
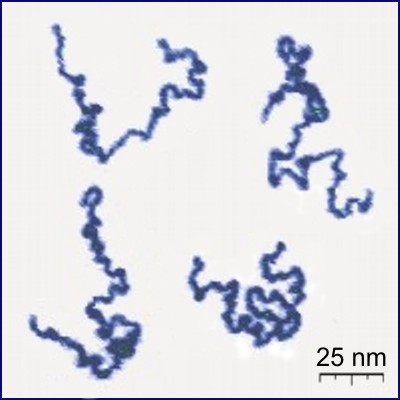
Polymers are macromolecules composed of many repeating units. Another way of seeing them is as long chains of atoms.
References:
Dove, M.T. (2002) Structure and Dynamics: An Atomic View of Materials Oxford Master Series in Condensed Matter Physics / Oxford University Press
Moore, J.T. (2010) Essential Chemistry for Dummies John Wiley & Sons
Simon, S.H. (2013) The Oxford Solid State Basics Oxford University Press
Author: César Tomé López is a science writer and the editor of Mapping Ignorance