Molecular mechanisms underlying the link between cannabis abuse and schizophrenia
Molecular mechanisms underlying the link between cannabis abuse and schizophrenia
Author: Leyre Urigüen is a researcher at the Department of Pharmacology, University of the Basque Country UPV/EHU and Centro de Investigación Biomédica en Red de Salud Mental CIBERSAM
Cannabis, also called marijuana, is a drug that comes from the plant Cannabis sativa (hemp plant). The plant contains more than 400 different chemicals, of which approximately 70 are considered cannabinoids. The major psychoactive component of the cannabis resin and flowers is called tetrahydrocannabinol (THC).
Cannabis is, by far, the most commonly used illicit drug across the world. Cannabis consumption especially in early adolescence, a period in which the brain is extremely vulnerable to its effects, increases the risk of developing schizophrenia.
Schizophrenia is a severe, chronic and disabling mental disorder that affects approximately 1% of the population worldwide. The symptoms of schizophrenia are classified into three categories: positive symptoms, negative symptoms and cognitive deficits. Positive or psychotic symptoms mainly include delusions (false beliefs), hallucinations (hearing voices) and thought disorder. Negative symptoms comprise social withdrawal, loss of motivation and a reduced capacity to express emotional states. Cognitive impairments include disturbances in working memory or language comprehension. The onset of the disease occurs in late adolescence and early adulthood.
Over the years, the question of whether cannabis is linked to the development of schizophrenia has frequently raised. Cannabis extracts can induce transient psychotic states in healthy subjects and worsen symptoms in schizophrenic patients. Moreover, the typical acute effects of cannabis resemble some of the symptoms of schizophrenia, in particular, the sensory distortions and loss of motivation.
To date, some epidemiological studies suggest that cannabis use increases the risk of developing schizophrenia but the neuronal and molecular mechanisms underlying psychotic symptoms elicited by cannabis abuse during adolescence remain unknown.
Brain serotonin receptors type 2A (5HT2A) seem to play a part in the molecular mechanisms responsible for psychotic symptoms. These receptors are the main target not only of the neurotransmitter serotonin but also of some hallucinogenic drugs like LSD (lysergic acid diethylamide) or DOI (dimethoxy-4-iodoamphetamine). In this context, the activation of these 5HT2A receptors in the brain after hallucinogenic drugs intake induces psychotic symptoms. These receptors, as well as many other receptors in the brain, are G-protein coupled receptors (GPCRs). This means that, once activated by its ligand, the receptor is going to recruit and activate a specific G-protein, which activates a cascade of further signalling events that finally results in a change in cell function. Nowadays, it is assumed that different ligands acting on the same receptor are able to activate different types of G-proteins (an event known as “biased agonism”), which finally results in the occurrence of different cellular changes. Regarding 5HT2A receptors, non-hallucinogenic ligands (i.e serotonin) exclusively activate the canonical pathway through Gαq/11-proteins. However, hallucinogenic 5HT2AR agonists, such as lysergic acid diethylamide (LSD) activate as well inhibitory Gαi/o proteins, leading to the unique effects of the hallucinogenic drugs.
In the last years, it has been suggested that the functionality of 5HT2A receptors is relevant for psychosis. For example, genetic studies have found polymorphisms in the 5HT2A gene in patients with schizophrenia. Animal models of schizophrenia have shown increased 5HT2A receptors functionality and it has been demonstrated that this receptor is up-regulated in the brain of patients with schizophrenia. Furthermore, some of the antipsychotics used for the treatment of schizophrenia act as antagonists of 5HT2A receptors, blocking its activation. All these data demonstrate that upregulation and/or increased functionality of 5HT2A receptors in the brain could predispose to psychosis or schizophrenia.
Considering these findings, we investigated1 whether chronic THC exposure at young ages could make the 5HT2A to recruit specific Gαi/o proteins, like the hallucinogenic drugs do, and therefore to induce psychosis.
For investigating this, we treated young mice (at a stage of brain development similar to that when human adolescence occurs) with high doses of THC (the psychoactive component of cannabis) during a long period (30 days) (trying to mimic the high potent cannabis consume in adolescents). After the treatment and before making any study, we left the animals free of THC for five days, for allowing the body to eliminate the entire drug from it.
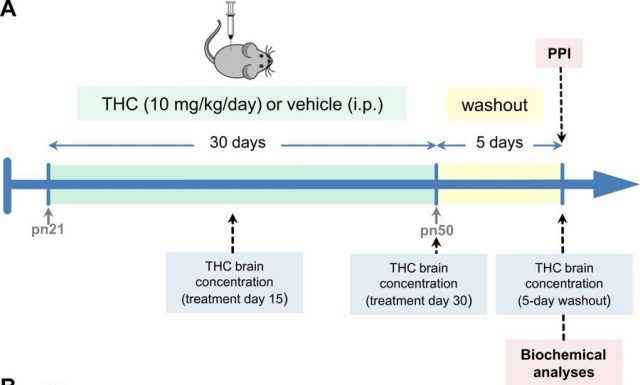
We first studied if THC administration was able to induce psychosis. For that purpose, we submitted the animals to the pre-pulse inhibition test (PPI). This test evaluates the behaviour of the mice and gives us information about the capability of the animals to integrate the sensorial (auditory) information. This very useful test has been widely used in patients with schizophrenia, who have alterations in their ability to filter and integrate the sensorial information. This PPI test has crossed from the clinical sphere to the basic research laboratories and results very useful for the evaluation of the psychotic-state of the mice.
What we found was that THC by itself was not able to induce psychosis in the mice. However, when we gave DOI (a hallucinogenic 5HT2A receptor agonist) to the animals previously treated with THC, they displayed much more psychosis than the animals treated only with DOI. It seemed like THC was somehow “preparing” the 5HT2A receptors to receive the hallucinogen drug DOI and potentiate its action. Therefore, we decided to study the status of 5HT2A receptors in the brain of these mice treated chronically with THC.
We used an experimental approach called SPA (scintillation proximity assay) that allows us to determine which of all G-proteins is recruiting the 5HT2A receptor when is activated by a ligand. Surprisingly, we found that when we gave the hallucinogen drug DOI, in mice treated with THC, the “hallucinogenic” intracellular pathway through Gαi/o proteins was potentiated while the “non-hallucinogenic” canonical pathway through Gαq/11-proteins remained unaltered.
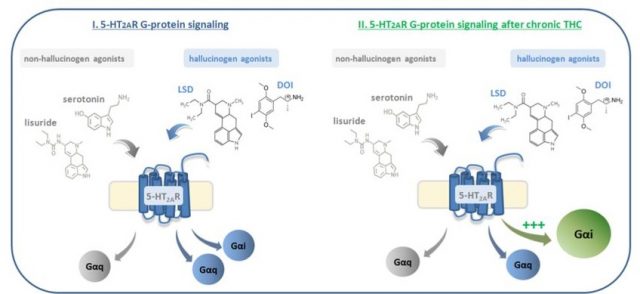
In summary, the present study demonstrates that chronic THC exposure leads to the over-activation of a pro-hallucinogenic signalling pathway of 5-HT2A receptors. Findings of the present work describe, for the first time, the molecular mechanisms underlying the link between cannabis abuse and susceptibility to schizophrenia.
References
- Ibarra-Lecue I, Mollinedo-Gajate I, Meana JJ, Callado LF, Diez-Alarcia R, Urigüen L. (2018) Chronic cannabis promotes pro-hallucinogenic signalling of 5-HT2A receptors through Akt/mTOR pathway. Neuropsychopharmacology doi: 10.1038/s41386-018-0076-y. ↩
1 comment
[…] El abuso del consumo de marihuana está relacionado con la aparición de síntomas de esquizofrenia. Ahora, Leyre Urigüen y su equipo han encontrado los mecanismos moleculares tras esta relación y ella misma nos lo explica en Molecular mechanisms underlying […]