The unexpected role of glycolaldehyde in photocatalytic cofactor regeneration using triethanolamine
Nature is a source of inspiration for scientists. If the efficiency of natural processes efficiency has been honed by billions of years of evolution, it seems reasonable that the best way to achieve some process is to try and mimic what nature has already come up with. This is the case with photosynthesis, a process that using sunlight as a the source of energy and carbon dioxide and water as raw materials can produce a huge variety of carbon-based chemicals or, from the technological point of view, an environment-friendly process that is able to store solar energy in the form of fuels, from organic molecules to hydrogen.
One way to look at photosynthesis is as a process where the energy of the sun is used to split the water molecule in order to release electrons that would be used to reduce an intermediate molecule, this molecule becoming an electron donor itself for the next part of the process where carbon from carbon dioxide would be itself reduced (the form that carbon has in most organic compounds). That intermediate molecule is NADP+.
NADP+ coenzyme is reducible to NADPH through binding of a proton and two electrons. This reduced form can then deliver the proton and electrons, potentially as a hydride, to reactions that culminate in the production of carbohydrates (the Calvin cycle). The coenzyme is recyclable in a natural photosynthetic cycle, but this process is yet to be artificially replicated.
This so-called regeneration of the cofactor molecule process needs another molecule that would be a sacrificial electron donor and, ideally, the energy needed would be provided by light. Triethanolamine (TEOA) has been extensively used for this purpose when the cofactor is nicotinamide adenine dinucleotide (NADH). The focus of research has been drawn on the development of photocatalysts. The efficiency of the catalysis depends on the electronic properties of the material (e.g., band gap energy) and the surface reaction sites that control the energy flow under the action of light. The list of suitable photocatalysts is relatively long and includes inorganic semiconductors, organic dyes, polymers, and plasmonic nanoparticles.
Virtually all the proposed photocatalysts follow the standard photoregeneration pathway that involves the light-induced generation of electrons and electron holes within the photocatalyst that drive the simultaneous NAD+ reduction and TEOA oxidation on the photocatalyst surface, respectively (Figure 1a).
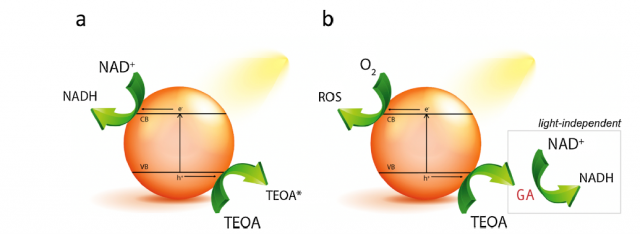
But it may not be that simple. Now, a team of researchers, including Ikerbasque researchersYury Rakovich (CFM & DIPC) and Marek Grzelczak (DIPC), shows 1 that, in contrast to the common model involving the light-induced electrons and holes generation to reduce the substrate and oxidize triethanolamine simultaneously, glycolaldehyde is the stable product of triethanolamine degradation capable of reducing NAD+ (Figure 1b).
The researchers provide experimental evidence that TEOA photo-oxidation and NAD+ reduction are not necessarily coupled processes. Actually, the pre-irradiation of TEOA solution in the presence of state-of-the-art photocatalysts including conjugated microporous polymer, graphitic carbon nitride (g-C3N4), platinum nanoparticles and titanium dioxide (TiO2) leads to the formation of glycolaldehyde, which induces NADH regeneration in the dark, even after the photocatalyst removal (Figure 1b).
On the other hand, NAD+ reduction to NADH by glycolaldehyde could be suppressed by lowering the pH below 8 or by the oxygen removal from the mixture. This suggests that TEOA, apart from its precursor role, also would maintain the required high pH of the mixture ensuring the reduction power of glycolaldehyde.
Apart for the better understanding of the mechanism underlying the cofactor regeneration in a version of artificial photosynthesis, the results of this basic research come with a bonus. They show that the photoactivation of the inexpensive TEOA leads to the production of a valuable chemical feedstock of relevance in organic chemistry and biology. For example, glycolaldehyde is an important intermediate in the formose reaction that involves the autocatalytic synthesis of biologically-relevant sugars (e.g. ribose) from formaldehyde as a starting material.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
References
- Karolina Kinastowska, Jie Liu; John M. Tobin, Yury Rakovich, Filipe Vilela Zhengtao Xu, Wojciech Bartkowiak and Marek Grzelczak (2019) Photocatalytic cofactor regeneration involving triethanolamine revisited: The critical role of glycolaldehyde Applied catalysys B: Evironmental doi: 10.1016/j.apcatb.2018.10.077 ↩
1 comment
[…] Eskala industrialean kopiatzeko interesgarrien den mekanismo naturaletako bat fotosintesia da. Ez da batere erraza eta pausu bakoitzean kontu berriak eta sorpresak daude. DIPC-koen The unexpected role of glycolaldehyde in photocatalytic cofactor regeneration using triethanolamine […]