Terminator: self-healing does no longer belongs to fiction only
About the author: Mikel Irigoyen is a PhD candidate at POLYMAT (UPV/EHU); his research is focused on the theoretical modelling of self-healing polymers.
As beautiful as it is, the natural world has always been a tough place to live in. Since their dawn, nearly 4 billion years ago, living organisms have faced a myriad of threats to their existence which have seen the majority of those who ever existed to perish. Only those organisms that managed to adapt to their environment by developing adaptive traits, like the wood frogs (Lithobates sylvaticus), who actually allow themselves to freeze over during hibernation allowing them to resist harsh Nordic winters, have been able to survive.
Similar to the natural world, the industrial world is also full of threats to its inhabitants. There are no frozen frogs inhabiting this world, but instead, there are plenty of materials crowding it, such as metallic ones like iron, aluminium or copper, wood, coming from different sources, minerals like quartz or granite, plastics, and polymeric materials. Not a single one of those is free of suffering on one way or another the harsh conditions in which they are used sometimes, meaning that their lifetime has an expiration date.
In this vein, loads of effort have been put in developing adaptive materials with the ability to repair the suffered damage as a response to external stimuli or even by themselves. This kind of materials are known as self-healing materials, and, by self-repairing the suffered damage, they have the ability to extend their lifetime.
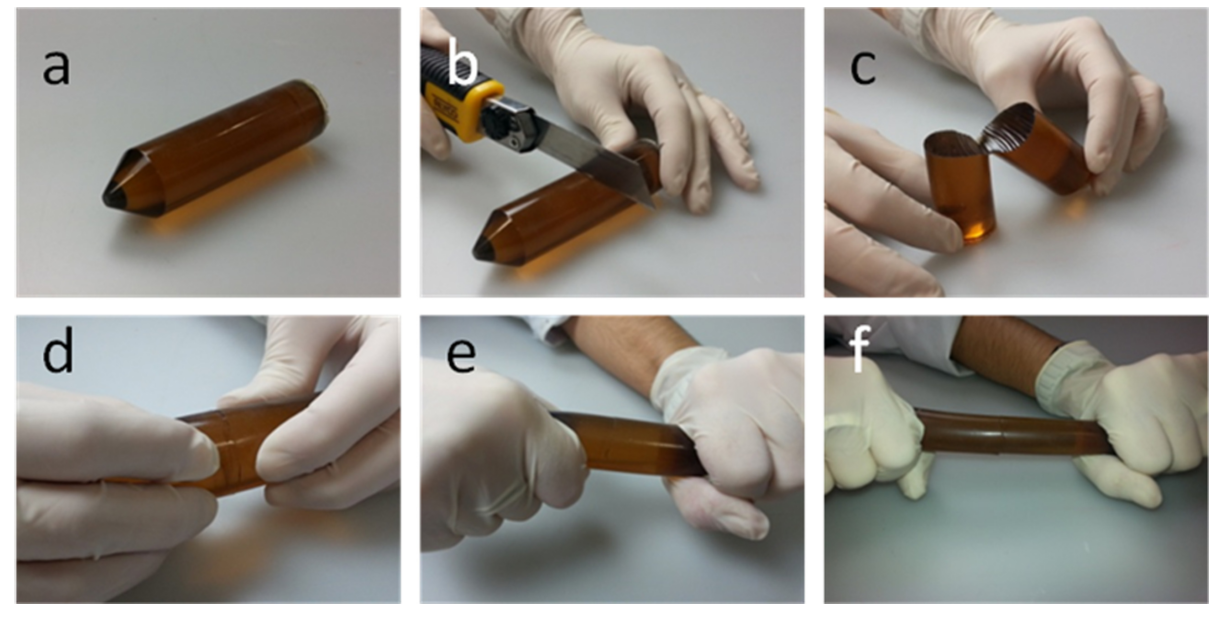
Among this family, we can find the so-called Terminator (Figure 1) which is a poly(urea-urethane) elastomer including aromatic disulfides in its network, and has the striking feature of self-healing the suffered damage at room temperature without the need of any external stimuli 1. The aforementioned aromatic disulfides were found responsible for this feature, and the underlying mechanism was studied by means of theoretical chemistry. This theoretical study 2 proved that the leading mechanism was a [2 + 1] radical-mediated mechanism, in which disulfide bonds are broken, forming sulfenyl radicals (which are reactive species). When formed, these radicals are able to attack the disulfide bonds of their neighbourhood, leading to an exchange reaction that supposes a rearrangement of the polymeric chains.
As a result of this study, it was possible to understand the factors which are controlling the whole process. As the exchange reaction, which is the first step of the self-healing process, involves the formation of radicals (i.e. the breaking of the disulfide bond), the first factor that needs to be assessed is the probability of radicals to be formed. Once the radicals are formed, they have to go through a reaction barrier, corresponding to the attack of a radical itself to a neighbour disulfide, in order to complete the exchange reaction. These barriers need to be rather small, because higher barriers would suppose that the reaction could not happen at room temperature, meaning that the energy needed to overpass the barrier would need to come from an external stimulus.
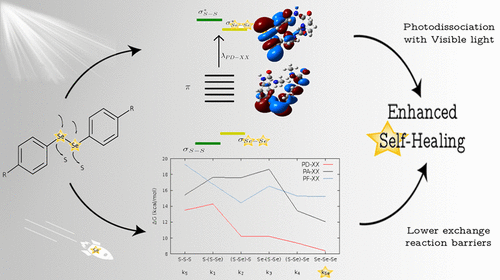
By knowing and understanding the “adaptive trait”of this particular family of materials, we are able to tailor brand-new materials who can outperform the already existing ones, similar to what happens in nature: evolution. In this vein, it was shown 3 that the inclusion of diselenide bonds instead of disulfide bonds can make the self-healing process more effective, as the reaction barriers are now lower (meaning that the exchange reaction needs lower energy). Moreover, it was also shown that, when aromatic diselenides are present, radicals could also be formed by absorption of visible light, a feature that is not common to aromatic disulfides.
So, when one thinks of Terminator, may be the well known line, “I’ll be back”, should be replaced by the Terminator singing the following brand-new version of Gloria Gaynor’s tune, I will survive:
Do you think I’d crumble,
Did you think I’d lay down and die?
Oh no, not I, I will survive
I will rearrange my chains, and form brand new disulfides
I will see my properties kept
And I won’t see myself swept, and I’ll survive
I will survive!
References
- Alaitz Rekondo, Roberto Martin, Alaitz Ruiz De Luzuriaga, Germán Cabañero, Hans J. Grande, and Ibon Odriozola. (2014) Catalyst-free room-temperature self-healing elastomers based on aromatic disulfide metathesis. Mater. Horizons, 1(2):237240 ↩
- Jon M. Matxain, José M. Asua, and Fernando Ruipérez. (2016) Design of new disulfide-based organic compounds for the improvement of self-healing materials. Phys. Chem. Chem. Phys. 18(3):17581770. ↩
- M. Irigoyen, A. Fernández, A. Ruiz, F. Ruipérez, and J.M. Matxain. (2019) Diselenide Bonds as an Alternative to Outperform the Efficiency of Disulfides in Self-Healing Materials. J. Org. Chem. 84(7).doi: 10.1021/acs.joc.9b00014 ↩