Engineering hybrid graphene nanoribbons with active electronic properties
Graphene nanoribbons (GNRs), are strips of graphene with ultra-thin width (<50 nm). Graphene ribbons, introduced as a theoretical model by Mitsutaka Fujita and coauthors to examine the edge and nanoscale size effect in graphene, have emerged as a promising material for nanoelectronics, as they combine many of the extraordinary properties of graphene with a high tunability of their electronic band structure.
In contrast to graphene, GNRs are frequently semiconducting materials, thus offering excellent perspectives for their utilization as electronic components such as diodes or transistors. The high susceptibility of those properties to minimum changes in the GNR structure means that they can be tuned through the precise control of their atomic building, but also indicates the stringent need for atomic precision in GNR synthesis.
The advent of bottom-up synthesis has open the path to defect-free GNRs, assembled by on-surface reactions of molecular organic precursors over a metal surface. These strategies rely on the careful design of suitable molecular precursors with specific shape and chemical composition to steer a step-wise reaction on a metal surface, leading to extended and atomically precise GNRs. An ample library of precursors and reaction pathways has been constructed in the last years, incorporating successful examples of precise control over the GNR’s width, orientation and edge topology.
Since most GNRs are semiconducting, a promising method for tuning their band structure is the electrostatic gating effect induced by doping. Chemical doping of GNRs has been achieved through the modification of the molecular precursors to either incorporate substitutional heteroatoms in the carbon backbone or by adding functional groups at the edges. In simplest scenarios, the effect of attached chemical groups can be described as an electrostatic gating of the native band structure.
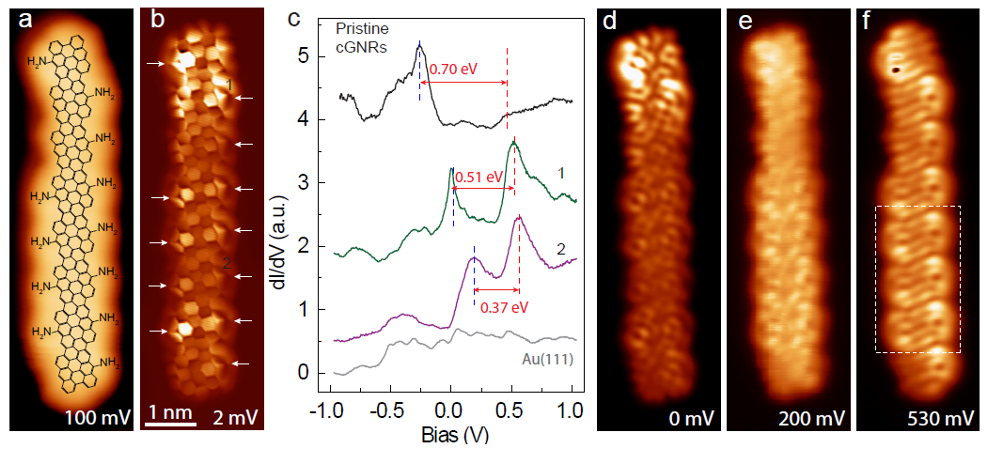
Now, a team of researchers shows 1 that amino (NH2) functional groups attached at the edges of chiral GNRs (chGNRs) lead to an upward shift of the electronic bands, with valence band crossing the Fermi level.
The amino functional groups were attached at the edges of narrow chiral GNRs with a sequence of 3 zigzag and 1 armchair sites ((3,1) chGNRs). NH2 end groups were substituted at the edges of pristine chGNRs through bottom-up synthesis on a Au(111) surface using functionalized bianthracene precursors.
A combination of scanning tunnelling microscopy and spectroscopy, and X-ray photoelectron spectroscopy measurements showed that the electron donating character of the amino group is inherited by the GNR, resulting in a valence band depopulation. The effective depopulation of the valence band depends on the number of NH2 groups per unit cell surviving the reaction, as well as on their relative alignment. Density functional theory simulations demonstrated that the enhanced electron-donating character of the doped ribbons arise from a substantial charge redistribution induced by the dopant.
These results indicate that a combination of edge terminations with different electron affinity character can be a promising route to engineer hybrid GNRs with active electronic properties.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may be copied verbatim or almost verbatim from the referenced research paper.
References
- Li JC, Brandimarte P, Vilas-Varela M, Merino-Diez N, Moreno C, Mugarza A, Mollejo JS, Sanchez-Portal D, de Oteyza DG, Corso M, Garcia-Lekue A, Pena D, and Pascual JI (2020) Band depopulation of graphene nanoribbons induced by chemical gating with amino groups ACS Nano doi: 10.1021/acsnano.9b08162 ↩