Graphene nanoribbons need to be protected from oxygen to remain functional
Real life is sometimes very different from laboratory settings. Take superconductivity, for example. In order to reach, it you either need extremely low temperatures, extremely high pressures or a combination of both; something that makes very difficult to conceive an everyday application out of a laboratory. Something similar happens with other new materials, but in their case the magic word is ultra low pressure, what we usually called a vacuum, in this case, ultra high vacuum. You can build almost anything, but in ultra high vacuum. Real life could deal with some vacuum, we use vacuum cleaners or Venturi pumps after all, but not with the ultra high requirement.
The interesting point is that studies about the effect of oxygen on the chemical structures that make some materials synthesized under ultra high vaccum conditions interesting are not that many. Take the case of graphene nanoribbons (GNRs), strips of graphene with ultra-thin width (<50 nm).
GNRs are very interesting structures, partly due to their attractive electronic properties. Those properties vary dramatically with changes in the nanoribbon’s atomic structure in terms of width, crystallographic symmetry, dopant heteroatoms, and edge termination. These electronic properties can be modulated even further by the appropriate design of GNR heterostructures. This remarkable tunability could mean a bright future as next-generation nanoelectronic and optoelectronic devices.
However, the high susceptibility of those properties to minimum changes in the GNR structure also indicates the stringent need for atomic precision in GNR synthesis. If oxygen of ambient conditions changes that structure, the limitations imposed on their use could make them no more than a basic science curiosity.
In fact, preliminary devices based on GNRs of various widths and armchair-oriented edges (aGNRs) have already been demonstrated, such as field-effect transistors and sensors. The advantage of aGNRs is that their edges are relatively unreactive, allowing for their synthesis directly in solution or, if synthesized in an inert vacuum environment, surviving transfer through ambient conditions at room temperature (and the rest of the processes associated with the device implementation) while still maintaining their electronic properties.
But this amounts almost to nothing as some of the most acclaimed properties of graphene nanostructures, like spin-split edge states – read spintronics, rely on the presence of zigzag edge segments. Examples include zigzag (zGNRs) and chiral GNRs (chGNRs), whose edges follow zigzag directions or alternate zigzag and armchair-oriented edge segments, respectively. The most reactive chemical species are radicals, species with unpaired electrons; well, a zigzag edge could have unpaired electrons in π orbitals that may behave as reactive radicals.
Previous experimental studies examining the effect of exposing GNRs to ambient conditions after their bottom-up synthesis on metallic surfaces under vacuum have been mainly focused on aGNRs. The results showed that, as we can expect, whereas the armchair edges are stable to such oxidizing conditions, the chemical stability of their zigzag termini is much more limited. On the other hand, theoretical studies have examined the potential effects of oxidizing zigzag edges. But nobody had checked what really happens in GNRs with a larger amount of zigzag edges under oxidizing conditions. Till now.
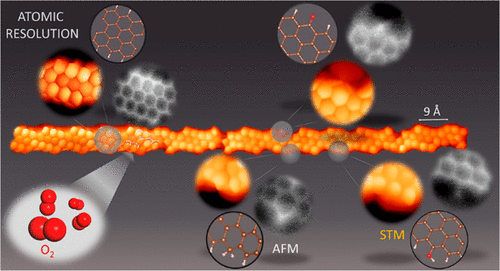
A team of researchers has used 1 a combination of bond-resolving scanning probe microscopy (BR-SPM), along with theoretical calculations to study (3,1)-chiral graphene nanoribbons [(3,1)-chGNRs] that were synthesized on a Au(111) surface and then exposed to oxidizing environments.
As could be expected, the exposure to the ambient atmosphere, along with the required annealing treatment to desorb a sufficiently large fraction of contaminants to allow for its post-exposure analysis by BR-SPM, revealed a significant oxidation of the ribbons, with the effect of ruining their electronic properties. Interestingly, more controlled experiments avoiding high temperatures and exposing the ribbons only to low pressures of pure oxygen show that also under these more gentle conditions the ribbons are oxidized. The scientists demonstrate that the oxidation is regioselective, with their most vulnerable spots at the central rings of the zigzag segments due to their higher radical character.
Therefore, we must conclude that graphene nanoribbons with zigzag edge segments require some form of protection before they can be used in or transferred through ambient conditions. Which ones is a good question.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research papers.
References
- Alejandro Berdonces-Layunta, James Lawrence, Shayan Edalatmanesh, Jesús Castro-Esteban, Tao Wang, Mohammed S. G. Mohammed, Luciano Colazzo, Diego Peña, Pavel Jelínek, and Dimas G. de Oteyza (2021) Chemical Stability of (3,1)-Chiral Graphene Nanoribbons ACS Nano doi: 10.1021/acsnano.1c00695 ↩