Are inorganic nanothreads possible? The case for borazine-based ones
Nanothreads are one-dimensional covalently bonded materials, with all “backbone” bonds saturated in the organic sense, the first of which was made from polymerization of benzene in the solid state. Nanothreads are thicker than conventional hydrocarbon polymers such as polyethylene and thinner than traditional nanowires: as ladder polymers, they are examples of the thinnest possible rigid objects.
Nanothreads generally form by pressure-induced room-temperature polymerization of multiply unsaturated molecules along stacks in certain crystallographic directions of the parent molecular crystal. The success in synthesizing nanothreads from diverse organic precursors reveals a great variety of structures and functionalities that may be accessible through choice of precursor molecule or cocrystal.
All nanothreads explored to date have carbon-based backbones. Are inorganic nanothreads possible? One possible approach here would be to replace all carbon dimers with BN pairs, thus yielding an isoelectronic BN-based nanothread; this strategy follows a logic, since hexagonal boron nitride (h-BN) sheets support important applications as insulating layers in multilayer 2D electronics. There is also a rich chemistry for BN analogues of all-C molecules.
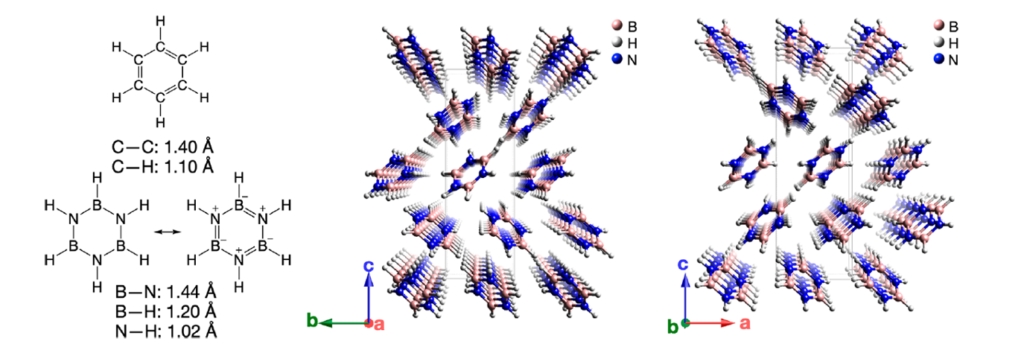
Borazine (B3N3H6) or “inorganic benzene” is isostructural and isoelectronic to benzene. As benzene forms organic nanothreads under pressure, one may anticipate that borazine can form BN nanothreads. Now, a team of researchers explores and identifies 1 all possible borazine-based nanothreads, the reaction mechanisms involved and calculates their polarization and piezoelectric response.
The large difference in electronegativity between boron and nitrogen produces an uneven distribution of π electron density that favors nitrogen. This nonuniform π system reduces aromaticity and makes borazine more reactive than benzene, consequently it can be anticipated that borazine may initiate polymerization at a lower pressure than benzene does, due to the reduced aromaticity.
In addition to the high mechanical strength and anisotropic thermal conductivity anticipated for carbon nanothreads, borazine nanothreads may also possess interesting electromechanical properties due to the heteropolar nature of the BN lattice in a highly anisotropic nanoscale structure with an
unusually stiff backbone.
The researchers examine borazine-derived nanothreads with degrees of saturation of 2, 4, and 6 (defined as the number of four-coordinated boron and nitrogen atoms per borazine formula unit) Nanothreads have been synthesized from benzene and pyridine, with evidence of both fully saturated (“degree-6”) and two-thirds saturated (“degree-4”). With the constraint of only two borazine formula units per topological unit cell, there are only 13 fully saturated (“degree-6”) BN nanothreads, only two of which are more stable than the borazine molecules. For comparison there are 50 such candidates for benzene-derived threads. This relative paucity of outcomes may assist in kinetic control of reaction products
The calculated polarization and piezoelectric response for the first four lowest-energy degree-6 BN threads are found to be modest compared with commercial bulk piezoelectric materials. Still, the distinct nanoscale organization lends itself to the induction of very large transfer strain gradients, due to the unusual combination of extreme narrowness and rigidity possible in nanothreads.
More on the subject:
Ideal furan and thiophene nanothreads
Highly ordered nanothread products using heteroatoms
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research papers
References
- Tao Wang, En-Shi Xu, Bo Chen, Roald Hoffmann, and Vincent H. Crespi (2022) Theory of Borazine-Derived Nanothreads: Enumeration, Reaction Pathways, and Piezoelectricity ACS Nano doi: 10.1021/acsnano.2c02778 ↩