Cancer cell clusters to foster metastatic spread
Author: José R. Pineda got his Ph.D. from University of Barcelona in 2006. Since 2007 he has worked for Institut Curie and The French Alternative Energies and Atomic Energy Commission. Currently, he is a researcher of the UPV/EHU. He investigates the role of stem cells in physiologic and pathologic conditions.
Cell migration is key to allow the cells to move between two points. This is of utmost importance during embryogenesis and development for proper organ formation. However, cell migration is another key characteristic that cancer cells use to invade and move from place to place exploring new environments and (in the worst case) colonizing them in a process that is called metastasis.
To date, two main modes of cell migration are known. 1) Movement that relies on contact of migrating cells with the extracellular matrix (ECM) or substrates through integrins and focal adhesions (small foot of cells used to contact with the external surfaces). Using this movement cells may travel independently or collectively using the mechanism of force generation to the ECM through focal adhesions, this mode converts cytoskeleton (branched-actin polymerization) into large protrusions and forward forces. 2) Amoeboid movement of single cells that is used as a propulsive locomotion that does not require specific adhesion and is driven by friction forces resulting from actomyosin retrograde flows.
However, the work published in Science Advances journal and led by Diane-Laure Pagès discovered, for the first time, a new type of cancer cell migration in confined non-adhesive (ECM-free) environment that empowers the cells to be able to move as cell clusters without the use of focal adhesions 1.
They started characterizing highly metastatic colorectal cancer cells observing the dissemination of cell-clusters in the lumen of lymphatic vessels without contact with ECM hypothesizing the possibility of a focal adhesion–independent mode of collective cell migration. To test this hypothesis, they fabricated non-chemotactic microchannels coated with an anti-adhesive polymer polyethylene glycol called “PEG” and performed a video track-recording up to 24h of primary explants of different types of digestive and genitourinary cancers. They found that cells moving packed migrated slowly compared to individual cells, but with a positive persistence (persistence is the tendency of a cell to continue moving in one direction).
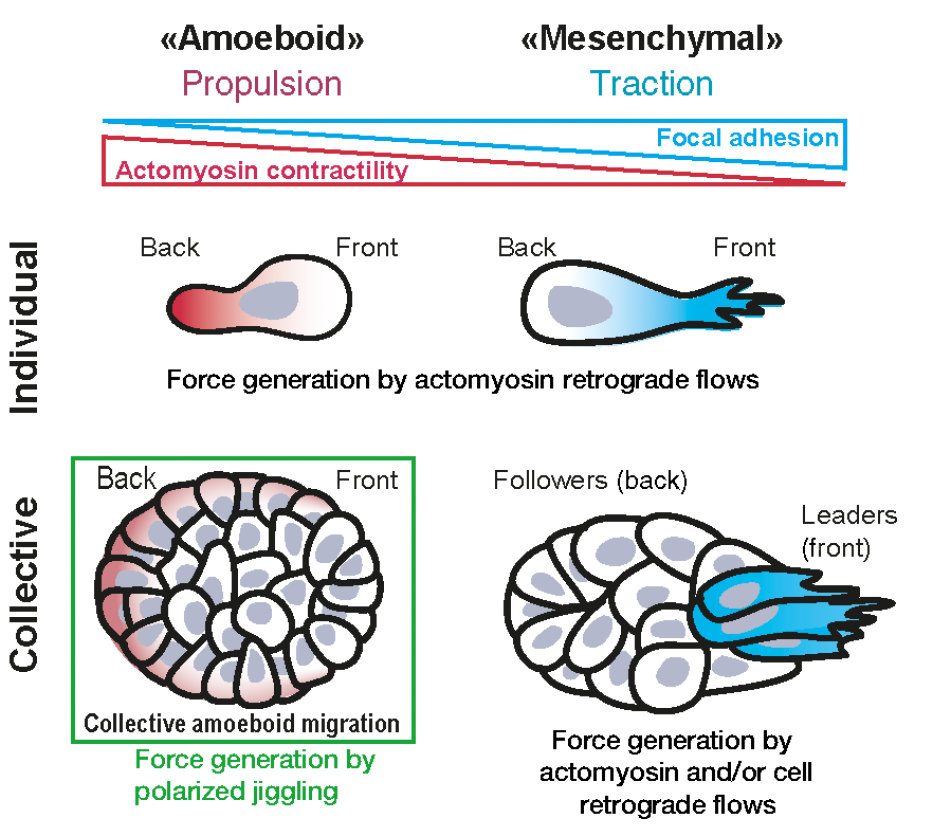
To discover the involved mechanism using genetic manipulation, they centered the studies in the HT29-MTX cell line. Firstly, they verified that non-adherent (ECM-free) cells migrated showing a rounded cell morphology meanwhile coating the microchannels with collagen (a protein characteristic of ECM) cells acquired the habitual spread shape. Then, to address the contribution of focal adhesions to cluster migration, they modified genetically the cells to express paxilin (a protein found in focal adhesions) tagged to a fluorophore observing that fluorescent paxillin revealed numerous contacts at the collagen-I interface (microchannel with ECM) but becoming nearly absent in PEG-coated (microchannels ECM-free) condition. To fully assess the participation of focal adhesions, the simple way is to inhibit them and observe the cellular response. To do that, they used pharmacological inhibitors (PF271, SU6656 and/or saracatinib) specific for two of their main cell signalling regulators: focal adhesion kinase and Src. They found that cell clusters in ECM-free condition exert no traction forces as opposed to localized pulling forces observed in collagen-I condition. At this point, it should be noted that integrins are also important components related to cell migration. Integrins transmit propulsive frictional forces against the substrate during single-cell amoeboid migration. Reducing its presence or functionality in cell membrane through its cell internalization or chemical inhibition, they found a reduction of velocity of cell migration. On the contrary, increasing cell-substrate friction adding to the microchannel a fine layer of BSA they found an increase of cell migration. These results concluded that cell clusters rely on integrin-mediated friction forces, migrating without focal adhesions in non-adhesive microchannels.
It is known that the cell regulates its contractility through actomyosin cytoskeleton. Actomyosin labelling through genetic modification to coexpress F-tractin and Turquoise-tagged myosin light chain showed a strong staining in median sections similar to those observed in primary tumor explants. Indeed, cell-cluster front/back polarity was positively correlated with migration speed, suggesting a “pro-migratory” function. Again, performing experiments of inhibition, they found that inhibiting myosin reduced cluster migration. However, the movement of the bulk of the cells may also be determined by the contractility of the rear of the cluster. To test whether increasing contractility of the cluster was sufficient to power their migration, they modified genetically using optogenetics to manipulate actomyosin contractility via its upstream regulator RhoA using the optoRhoA system. Using this strategy, they induced the contractility, deciding the specific region and time using light exposure. They illuminated the different parts of the migrating clusters and monitored their trajectories for up to 10 hours and find out that activation at the rear did not yield a significant increase of speed of migration but increasing actomyosin activity in a subset of cells was able to dictate the direction of migrating clusters.
At this stage, the researchers prompted the question if the contractility supported collective and single-cell migration by orienting the retrograde flows of cells, then cell placement over time in the cluster could be implied in cluster movement. To answer this question, they modified genetically some cells of the cluster, labelling them with red fluorescent protein (RFP)–tagged histone 2B (histones are proteins found in the chromatin inside of cell nucleus). After video-recording for 11 hours and analyzing the results, they concluded that clusters move cohesively as solids in the absence of sustained retrograde cellular flows.
In light of previous conclusions and having discarded the possibility of cell movement inside the cluster, another question that arose was the possibility that cluster migration could be done associated with fluctuating cell deformations and/or myosin/nuclei speed (this last sort of movements is called “jiggling”). The researchers determined the amplitude of myosin flow or nuclei speed fluctuations at the contact with the channels walls, the fluctuations of nuclei displacements observed in lateral cells and the fluctuations throughout the cluster. They found more movement of these structures in fast moving clusters. Therefore, they concluded that the jiggling was positively correlated with cluster migration speed.
In conclusion, Diane-Laure Pagès and collaborators found a new mode of migration (Figure 1) that they named “collective amoeboid”. Contrary to previous characterizations of cell migration, they identified random fluctuations of myosin flows based on polarized jiggling that yields directed motion in non-adhesive environments producing cell displacements in migrating clusters. This new mode of migration may be underlying the migration of cancer cell clusters to disseminate through perimuscular tracks, the lumen of lymphatic vessels, or the peritoneal and pleural cavities among others. Indeed, in the course of cancer metastatic dissemination, tumor cells hijack all modes of single or collective migration to take advantage and this resembles the capacity of white blood-cells leukocytes to move and explore all kinds of environments using the integrin-independent mode of migration to assure to reach and colonize all new environments.
References
- Diane-Laure Pagès, Emmanuel Dornier, Jean de Seze, Emilie Gontran, Ananyo Maitra, Aurore Maciejewski, Li Wang, Rui Luan, Jérôme Cartry, Charlotte Canet-Jourdan, Joël Raingeaud, Grégoire Lemahieu, Marceline Lebel, Michel Ducreux, Maximiliano Gelli, Jean-Yves Scoazec, Mathieu Coppey, Raphaël Voituriez, Matthieu Piel, Fanny Jaulin. (2022) Cell clusters adopt a collective amoeboid mode of migration in confined nonadhesive environments. Sci Adv. doi: 10.1126/sciadv.abp8416. ↩