Size-dependent glass transition in metallic glasses
We all know what glass is, don’t we? We just point to the nearest window and it is that transparent sheet. Humans have been producing glass for at least 6,000 years, way before they knew how to smelt iron. Actually, the chemistry is well known: to produce window glass we just heat a mixture of sodium carbonate (soda), calcium oxide (lime) and silicon (IV) oxide (silica or, less technically, sand) and we have soda glass. If we want our glass to withstand temperature changes and be tougher, we add some boron oxide and, instead of a calcium silicate, we get a borosilicate with a fancy name (Pyrex). We can add other elements to get other special characteristics in our glass. It is as if we knew what we are doing. But we are not.
The glass transition or vitrification, understood as a good theoretical description of the transformation of a liquid, supercooled below its melting temperature, into a glass, is one of the most fascinating and still unsolved problems in condensed matter physics. Besides the underlying fundamental process, the way vitrification actually takes place and how old the glass is can deeply impact the glass properties and their evolution.
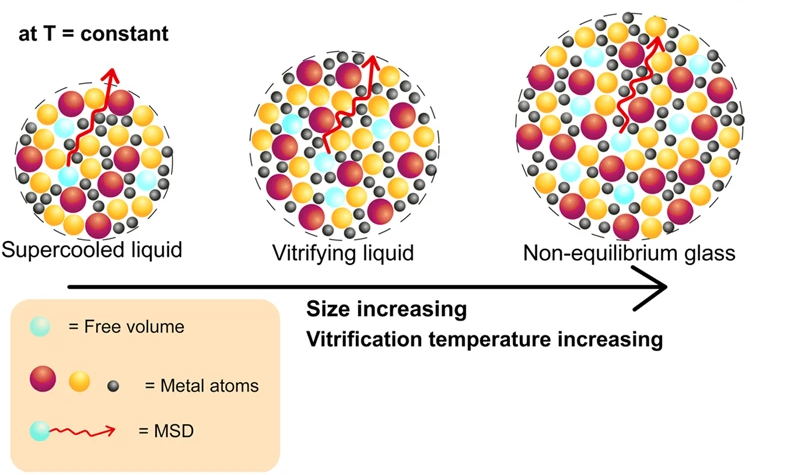
Among the variety of glasses, a class of utmost importance from both fundamental and technological viewpoints is that of metallic glasses. They combine different technological relevant properties such as superior mechanical properties and corrosion resistance, which could be deeply affected by how vitrification has taken place. For instance, the fracture toughness of metallic glasses can be varied by vitrifying at different cooling rates. This is described by the concept of fictive temperature, Tf , defined as the temperature at which the supercooled liquid falls out of thermodynamic metastable equilibrium.
Conventional wisdom describes vitrification as triggered exclusively by the main, α, relaxation. Recently, it has been shown for an Au-based metallic glass that vitrification at low cooling rates results in values of Tf lower than those which would be obtained accounting for the α relaxation only. This means that atomic mechanisms different from the α relaxation may be important actors in vitrification kinetics.
Beside the role of different mechanisms in vitrification kinetics, a long-standing problem concerns whether vitrification and atomic/molecular relaxation can be modified by reducing the sample size. When the concept of Tf is employed to characterize vitrification kinetics, significant reductions have been found for polymer samples with size exceeding the micrometer length scale. While deeply investigated in glass-forming polymers, whether reducing the sample size in metallic glasses may be of relevance in affecting vitrification kinetics has so far remained completely elusive and largely unexplored. One of the most intriguing observations is that a gradual change in fracture morphology of a Pt-based metallic glass from vein-pattern to completely smooth fracture surface to necking is observed with decreasing sample size at micrometer length scales and testing temperature.
Now, a team of researchers thoroughly investigate 1 size-dependent glass transition in two archetypal metallic glasses, based on Au and Pt, respectively.
For this purpose, the researchers employ fast scanning calorimetry permitting a large range of heating/cooling rates from 0.5 K/s up to 5000 K/s. In samples prepared in identical conditions, look into both atomic mobility, that is the rate of spontaneous fluctuations taking place in the unperturbed supercooled liquid at equilibrium, and vitrification kinetics in a wide range of time scales.
The team finds that atomic mobility remains bulk-like for all investigated sample sizes, ranging from bulk to several microns. In contrast, they observe pronounced size-dependent vitrification kinetics, more evident for the smallest samples and at low cooling rates. As a result, vitrification of metallic glasses takes place at temperatures lower than bulk for samples size below ~10 μm.
The important implication of this outcome is that mild reductions of the sample size in a metallic glass would allow exploring thermodynamic states, in terms of Tf, deep down in the energy landscape, thus opening the door to obtaining a thermodynamically ultra-stable metallic glass in time scales amenable to the experimental practice. These results even bear potential to convey insights on the existence of the ideal glass.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research paper/s.
References
- Valerio Di Lisio, Isabella Gallino, Sascha Sebastian Riegler, Maximilian Frey, Nico Neuber, Golden Kumar, Jan Schroers, Ralf Busch & Daniele Cangialosi (2023) Size-dependent vitrification in metallic glasses Nature Communications doi: 10.1038/s41467-023-40417-4 ↩