The secret structures of benzodithiophene polymers unlock solar energy
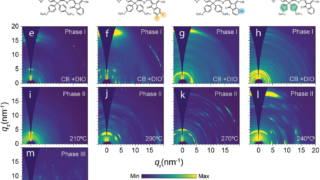
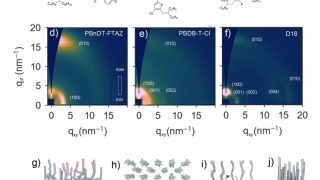
Organic electronics in the form of organic solar cells — thin, flexible, and printable on everyday surfaces — promise a sustainable future in renewable energy. Unlike traditional silicon-based solar panels, organic photovoltaic (OPV) devices rely on carbon-based polymers, a class of molecules more commonly associated with plastic bags than power generation. Among these, a family […]