Rejuvenation through the eradication of accumulating senescent cells
Author: José R. Pineda got his Ph.D. from University of Barcelona in 2006. Since 2007 he has worked for Institut Curie and The French Alternative Energies and Atomic Energy Commission. Currently, he is a researcher of the UPV/EHU. He investigates the role of stem cells in physiologic and pathologic conditions.
Senescence can be defined as the condition or process of deterioration with age. Cellular senescence is a stress response program characterized by both cell cycle arrest due to progressive cellular and DNA damage and the change of the secretory program towards the release of pro-inflammatory cytokines (secreted substances that have an effect on other cells). It is already known that during aging, this cellular progressive damage and the lack of the proper role of immune cells clearing the senescent cells lead to the accumulation of the latter, thus increasing the pro-inflammatory signals in our body. Current strategies to screen and eliminate senescent cells are based on the repeated administration of drugs over time.
The work of Corina Amor and collaborators published in the prestigious journal Nature Aging focuses on a new strategy, taking advantage of the immune system’s training and learning tools known as “CAR T” (acronym of “Chimeric antigen receptor (CAR) T-cell therapy”). This therapy is based on the harvesting of immune cells called T cells (a type of white blood cell) from the patient using a procedure called leukapheresis. Next and most important is to carry out the modification of these T cells in a lab by adding the gene for the specific chimeric antigen receptor (CAR) of interest, amplify (or grow) these new cells, and then infuse them back into the patient. For example, this strategy is currently known as “immune therapy against cancer cells” when the antigen that these T cells learn to recognize is specific to cancer cells. Corina Amor et al. used this already-known technology to focus its utility on targeting and destroying senescent cells instead of tumor cells 1.
In order to be able to reorganize tissue, the old and senescent tissue must be able to be degraded. An important mechanism in this degradation is the proteolysis cascade initiated by the plasminogen activation system. Thus, senescent cells upregulate the cell-surface receptor of urokinase plasminogen activator (uPAR), also known as CD87. By modifying CAR T cells to target uPAR, Corina Amor and collaborators demonstrated an efficient strategy to deplete senescent cells in aged mice and indirectly modulate their health span.
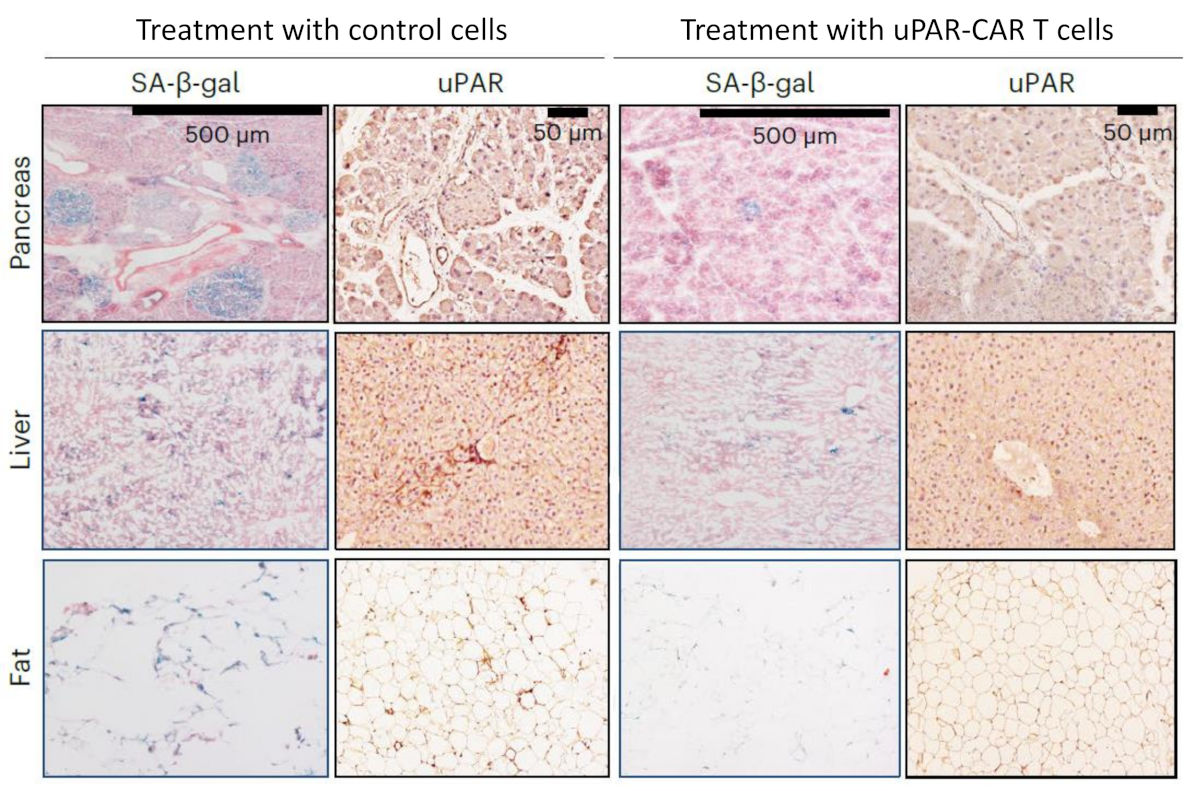
Firstly, to explore the correlation of uPAR levels in aged tissues, they analyzed RNA-sequencing data (RNA-seq) between young mice (3-month-old) and elder mice (20-month-old). They found that Plaur, the encoding gene of uPAR, was upregulated in older animals. Next, they corroborated the finding using immunohistochemistry, confirming the increased expression of uPAR protein in the liver, adipose tissue, skeletal muscle, and pancreas, correlating its co-expression with the senescent marker beta-galactosidase (SA-β-gal). To corroborate this finding, they did FACS cell sorting to separate uPAR positive and negative cell populations for single-cell RNA sequencing, observing that endothelial cells (cells that cover the inner surface of our vasculature) were the most prominent uPAR-expressing populations in both the liver and pancreas. Furthermore, uPAR-positive cells were significantly enriched in gene signatures linked to inflammation, the complement pathway, and the coagulation cascade, as well as transforming growth factor beta signaling (TGF): another cell signaling pathway that increases in endothelial cells during aging that I discovered to have a direct role in neural stem cell quiescence 23. Corina Amor and collaborators also extrapolated the relevance of their findings in human tissue by comparing available datasets of human pancreas collected from young (0–6-year-old) and aged (50–76-year-old) individuals, observing again the increase of PLAUR in older individuals.
Next, they decided to determine the tolerability and therapeutic activity of uPAR-targeting CAR T cells (m.uPAR-m.28z CAR T) or unstransduced T cells (control), administering 0.5×106 cells intravenously for each aged mouse. They found a reduction in the proportions of SA-β-gal-positive and uPAR-positive cells throughout the tissues examined, as well as a reduction in the levels of pro-inflammatory cytokines, without significant changes in morbidity, weight loss, blood counts, or alterations in serum chemistry. Because the most frequent age-related metabolic dysfunctions in humans are impaired glucose tolerance and decreased exercise capacity, they analyzed glucose levels in mice that had previously received an intraperitoneal bolus of glucose (2 g/Kg-1 body weight), observing a reduction of plasma glucose levels for over two hours after administration in CAR T treated mice. Interestingly, CAR T treated mice also showed a lower basal insulin level and a strong increase 15 min after glucose administration, indicating improved pancreatic beta cell function. Moreover, CAR T cell-treated old mice showed improvements in their exercise capacity at 2.5 months after treatment compared to pretreatment levels.
After the observation of these promising results, the next question was to determine the persistence of the intravenously infused cells over time. To solve this question, they infused CAR T cells in 3-month-old young animals and monitored the mice over their natural lifespan, detecting the cell presence up to 12-months post-infusion (note that a mouse lives about 24 months). These results demonstrated that prophylactic CAR T cell administration in young mice was able to limit the metabolic decline in old age as they had significantly lower fasting glucose levels, improved glucose tolerance, and higher exercise capacity.
Finally, they decided to do the opposite approach: reproduce metabolic alterations characteristic of old mice in young mice and test the efficiency of CAR T cell therapy. To do so, they administered a high-fat diet to young animals to induce obesity (obesity has been described as accelerating the ‘aging clock’). They observed an accumulation of senescent cells in these young animals. After two months of this high-fat diet, the animals received a treatment of 0.5×106 CAR T cells and continued the diet, observing a significant lower body weight, reduction of senescent cell populations, better fasting blood, and improvements in both glucose and insulin tolerance in the cohort infused with uPAR-CAR T compared to the control that were sustained for 5.5 months after cell infusion. In conclusion, uPAR CAR T cell therapy produced a similar improvement to metabolic dysfunction in the context of metabolic syndrome in young animals, as was observed in naturally aged mice.
In conclusion, the results of this work show that uPAR CAR T cells can safely and effectively remove senescent uPAR-positive cells from the tissues of naturally aged mice and ameliorate age-dependent metabolic and physical dysfunction. These discoveries pave the way for the use of the CAR T strategy in the future identification of tissue-specific senolytic antigens that could be targeted with cellular therapy to treat different age-dependent conditions.
References
- Amor C, Fernández-Maestre I, Chowdhury S, Ho YJ, Nadella S, Graham C, Carrasco SE, Nnuji-John E, Feucht J, Hinterleitner C, Barthet VJA, Boyer JA, Mezzadra R, Wereski MG, Tuveson DA, Levine RL, Jones LW, Sadelain M, Lowe SW. (2024) Prophylactic and long-lasting efficacy of senolytic CAR T cells against age-related metabolic dysfunction Nat Aging doi: 10.1038/s43587-023-00560-5 ↩
- Pineda JR, Daynac M, Chicheportiche A, Cebrian-Silla A, Sii Felice K, Garcia-Verdugo JM, Boussin FD, Mouthon MA. (2013) Vascular-derived TGF-β increases in the stem cell niche and perturbs neurogenesis during aging and following irradiation in the adult mouse brain EMBO Mol Med. doi: 10.1002/emmm.201202197 ↩
- Pineda JR, Boussin FD, Mouthon MA. (2013) TGFβ, a troublemaker in the adult neural stem cell niche Med Sci (Paris). doi: 10.1051/medsci/2013296006 ↩