Subcortical nuclei and language processing
Authors: Elissa-Marie Cocquyt, speech therapist and postdoctoral researcher at the University of Groningen & Adrià Rofes, assistant professor of Neurolinguistics at the University of Groningen.
The human brain is one of the most wonderful organs. In general, our brain consists of a left and right part, of which the outermost layer is called the ‘cortex’. The functions of the cortex have been extensively investigated and comprise all aspects of human behavior, from walking to your car and driving to the grocery store to remembering the name of your neighbor and the ability to chat with him 1. An accurate execution of these daily activities relies on key interactions between the cortex and little structures in the depth of the brain: the ‘subcortical nuclei’ 2. In this article, we will delve into the intricacies of the subcortical nuclei. In particular, we will emphasize their potential contribution in language processing. We will do so, by targeting a specific patient population, i.e. Parkinson’s disease (PD), and a treatment option, i.e. Deep Brain Stimulation (DBS).
PD is the second most common neurodegenerative disease worldwide, after Alzheimer’s Disease 3. It is typically described as a motor disorder due to the presence of a resting tremor, rigidity, slower/smaller movements, and postural imbalance 4. Importantly, PD is not limited to motor symptoms. Sleep and smell disorders, fatigue, anxiety and depression (to name a few) can also be observed, sometimes even before the onset of the motor symptoms 5. PD has an important impact on the communicative abilities of patients as well. This is partially due to the speech1 problems they experience, typically including a reduction in loudness, pitch variations and articulation precision. These symptoms are related to the motor symptoms mentioned before 6. In addition, PD can compromise language2 production and comprehension.
Specific to language, frequently reported symptoms are (1) difficulties in complex (e.g. embedded3) sentence comprehension (e.g. 7 8), (2) a reduced accuracy (compared to healthy controls) on verbal fluency (i.e. generating as much words from a specific category as possible in a given time) and picture naming tasks, especially when action verbs (e.g. jumping, writing, running etc.) are targeted (e.g. 9 10 11) and (3) less information transfer during spontaneous language production (story retelling or picture description) tasks, meaning that less of what they say is considered informative (e.g. 12 13).
Equally important, the presence of cognitive (e.g. attention, inhibition, flexibility, working memory) disorders can heavily influence the results obtained during language tasks. Indeed, in current research, it remains an open question whether the observed language difficulties can be fully explained by the presence of cognitive disorders 14.
Looking into the brain of people with PD, one of the key characteristics is a significant loss of specific (‘dopaminergic’) nerve cells in the substantia nigra (a subcortical nucleus), which on his turn influences the interactions with other subcortical structures and the cortex. Disturbances in these interactions give rise to the multitude of symptoms observed in people with PD 15. Until today, there is no definite cure for PD. Hence, the available treatment options focus on relieving and controlling the (motor) symptoms, by influencing the disturbed brain interactions. As a first treatment, many patients will receive medication (e.g. levodopa), which is supposed to compensate for the loss of dopaminergic nerve cells 16.
For those individuals in whom medication effects become unpredictable, DBS surgery can be considered. This is a medical procedure during which electrodes are implanted in specific subcortical nuclei. In case of PD, the target region for electrode placement frequently is the subthalamic nucleus (STN) in both the left and right part of the brain (Figure 1). The electrodes are connected by leads with a neurostimulator, which, on his turn, is implanted in the abdominal wall or chest. Depending on the neurostimulator settings, an electrical current can be sent to the STN 17 18.
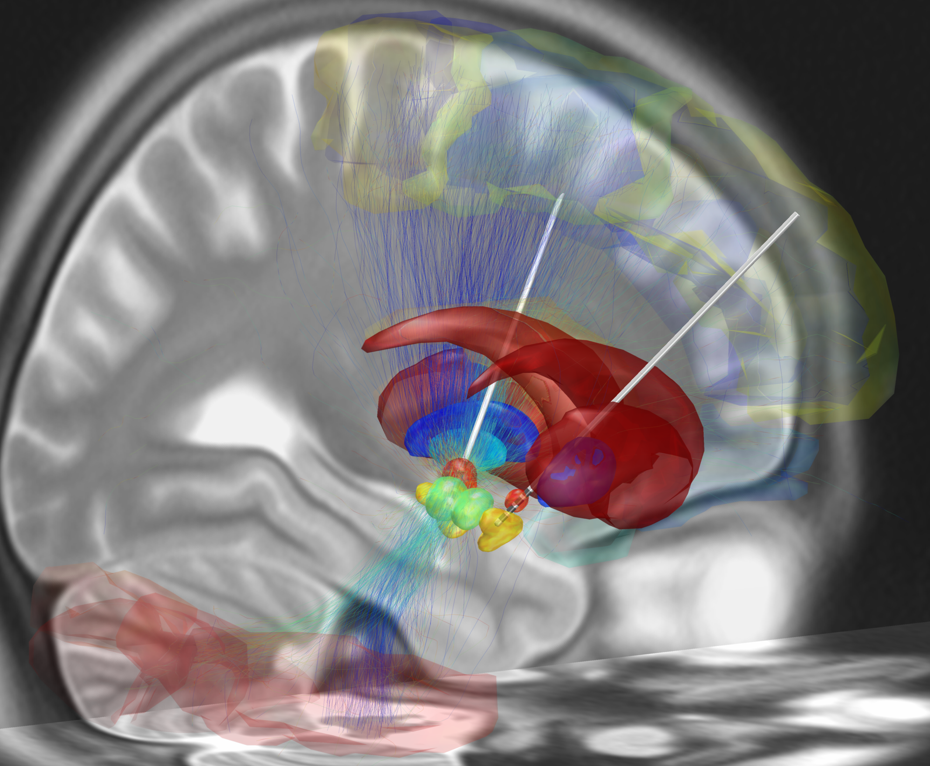
DBS surgery and STN stimulation result in a long-term improvement of the motor symptoms in people with PD, and allow a reduction of medication treatment 19. Nonetheless, the effects of STN DBS on language functioning are less clear. In general, most studies comparing language functions before and after DBS surgery focused on verbal fluency tasks, and reported a decline in performance (weeks to months) after surgery (for a review, see 20). Fewer follow-up studies made use of other language tasks, which is an important limitation. In these studies, the effects of STN DBS were variable: both stable performances (e.g. for picture naming 21), accuracy improvements (e.g. for spontaneous language production, 22) and declines (e.g. on decision tasks targeting words with multiple meanings (e.g. ‘table’ 23) have been observed after (compared to before) DBS surgery.
These results provide evidence that the STN may be part of the brain networks responsible for language functions, but also show that the effect of STN DBS varies according to the type of task 24. Importantly, however, caution is needed when comparing the results of different studies due to the many differences in their approach (method), and differences between patients. For example, the decision when to perform the language evaluation (before and after surgery) could influence the obtained results: Moretti et al. 25 showed that it could take up to twelve months after surgery before a beneficial effect of STN DSB on complex sentence comprehension is observed. Also, the specific characteristics of each individual patient (e.g. disease duration and severity, medication intake, STN stimulation parameters, cognitive abilities) can give rise to unique patterns of language functioning, and unique effects of STN DBS 26.
In summary, the multitude of symptoms in people with PD, and the diverse effects of DBS stimulation, make it clear that subcortical brain structures, although small in size, play a big role in human functioning. Focusing on communication, exciting research lines on language processing (beyond verbal fluency), the link with cognition, as well as the long-term effects of DBS in this population are underway 27. Going in depth (of the brain) can lead to a better understanding of the complex interplay between the cortex and subcortical nuclei, and provide clinicians with better tools to counsel people with PD.
Acknowledgements
Both authors received funding from the Dutch Research Council (NWO, 406.XS.01.050).
Notes
1 Speech refers to the motor act of talking. Important elements are the articulation, voice quality and speech rhythm (American Speech-Language-Hearing Association, https://www.asha.org/public/speech/development/speech-and-language/).
2 Language refers to the non-motor act of talking, namely how we receive (understand) and use (produce) words. Important elements are the meaning of words, the formation of words (e.g. friend, friends, friendly) and placing words in the correct order to form a sentence (American Speech-Language-Hearing Association, https://www.asha.org/public/speech/development/speech-and-language/).
3 Example of an embedded sentence: “The boy, who won the race, received a medal.”
.
References
- Swenson, R. S., & Gulledge, A. T. (2017). The cerebral cortex. In Conn’s Translational Neuroscience (pp. 263-288). Academic Press. ↩
- Alexander, G. E., Crutcher, M. D., & DeLong, M. R. (1991). Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Progress in Brain Research, 85, 119-146. ↩
- De Lau, L. M., & Breteler, M. M. (2006). Epidemiology of Parkinson’s disease. The Lancet Neurology, 5(6), 525-535. ↩
- Jankovic, J. (2008). Parkinson’s disease: clinical features and diagnosis. Journal of Neurology, Neurosurgery & Psychiatry, 79(4), 368-376. ↩
- Poewe, W., Seppi, K., Tanner, C. M., Halliday, G. M., Brundin, P., Volkmann, J., … & Lang, A. E. (2017). Parkinson Disease. Nature reviews Disease primers, 3(1), 1-21. ↩
- Duffy, J. R. (2013). Motor speech disorders: Substrates, differential diagnosis, and management: Elsevier Health Sciences ↩
- Grossman, M., Glosser, G., Kalmanson, J., Morris, J., Stern, M. B., & Hurtig, H. I. (2001). Dopamine supports sentence comprehension in Parkinson’s disease. Journal of the Neurological Sciences, 184(2), 123-130. ↩
- Hochstadt, J., Nakano, H., Lieberman, P., & Friedman, J. (2006). The roles of sequencing and verbal working memory in sentence comprehension deficits in Parkinson’s disease. Brain and language, 97(3), 243-257. ↩
- Bocanegra, Y., García, A. M., Lopera, F., Pineda, D., Baena, A., Ospina, P., … & Cuetos, F. (2017). Unspeakable motion: Selective action-verb impairments in Parkinson’s disease patients without mild cognitive impairment. Brain and language, 168, 37-46. ↩
- Cotelli, M., Borroni, B., Manenti, R., Zanetti, M., Arévalo, A., Cappa, S. F., & Padovani, A. (2007). Action and object naming in Parkinson’s disease without dementia. European Journal of Neurology, 14(6), 632-637. ↩
- Signorini, M., & Volpato, C. (2006). Action fluency in Parkinson’s disease: A follow‐up study. Movement disorders: official journal of the Movement Disorder Society, 21(4), 467-472. ↩
- Murray, L. L. (2000). Spoken language production in Huntington’s and Parkinson’s diseases. Journal of Speech, Language, and Hearing Research, 43(6), 1350-1366. ↩
- Roberts, A., & Post, D. (2018). Information content and efficiency in the spoken discourse of individuals with Parkinson’s disease. Journal of Speech, Language, and Hearing Research, 61(9), 2259-2274. ↩
- Lowit, A., Thies, T., Steffen, J., Scheele, F., Roheger, M., Kalbe, E., & Barbe, M. (2022). Task-based profiles of language impairment and their relationship to cognitive dysfunction in Parkinson’s disease. PLoS One, 17(10), e0276218. ↩
- Rietdijk, C. D., Perez-Pardo, P., Garssen, J., Van Wezel, R. J., & Kraneveld, A. D. (2017). Exploring Braak’s hypothesis of Parkinson’s disease. Frontiers in Neurology, 8, 37. ↩
- Salat, D., & Tolosa, E. (2013). Levodopa in the treatment of Parkinson’s disease: current status and new developments. Journal of Parkinson’s disease, 3(3), 255-269. ↩
- Kleiner‐Fisman, G., Herzog, J., Fisman, D. N., Tamma, F., Lyons, K. E., Pahwa, R., … & Deuschl, G. (2006). Subthalamic nucleus deep brain stimulation: summary and meta‐analysis of outcomes. Movement disorders: official journal of the Movement Disorder Society, 21(S14), S290-S304. ↩
- Okun, M. S. (2012). Deep-brain stimulation for Parkinson’s disease. New England Journal of Medicine, 367(16), 1529-1538. ↩
- Evidente, V. G. H., Premkumar, A. P., Adler, C. H., Caviness, J. N., Driver‐Dunckley, E., & Lyons, M. K. (2011). Medication dose reductions after pallidal versus subthalamic stimulation in patients with Parkinson’s disease. Acta Neurologica Scandinavica, 124(3), 211-214. ↩
- Vos, S. H., Kessels, R. P., Vinke, R. S., Esselink, R. A., & Piai, V. (2021). The effect of deep brain stimulation of the subthalamic nucleus on language function in Parkinson’s disease: A systematic review. Journal of Speech, Language, and Hearing Research, 64(7), 2794-2810. ↩
- Heo, J. H., Lee, K. M., Paek, S. H., Kim, M. J., Lee, J. Y., Kim, J. Y., … & Jeon, B. S. (2008). The effects of bilateral subthalamic nucleus deep brain stimulation (STN DBS) on cognition in Parkinson disease. Journal of the Neurological Sciences, 273(1-2), 19-24. ↩
- Zanini, S., Melatini, A., Capus, L., Gioulis, M., Vassallo, A., & Bava, A. (2003). Language recovery following subthalamic nucleus stimulation in Parkinson’s disease. Neuroreport, 14(3), 511-516. ↩
- Whelan, B. M., Murdoch, B. E., Theodoros, D. G., Hall, B., & Silburn, P. (2003). Defining a role for the subthalamic nucleus within operative theoretical models of subcortical participation in language. Journal of Neurology, Neurosurgery & Psychiatry, 74(11), 1543-1550. ↩
- Murdoch, B. E., & Whelan, B-M. (2009). Speech and language disorders associated with subcortical pathology. Wiley-Blackwell. ↩
- Moretti, R., Torre, P., Antonello, R. M., Capus, L., Marsala, S. Z., Cattaruzza, T., … & Bava, A. (2003). Neuropsychological changes after subthalamic nucleus stimulation: a 12 month follow-up in nine patients with Parkinson’s disease. Parkinsonism & Related Disorders, 10(2), 73-79. ↩
- Whelan, B. M., Murdoch, B. E., Theodoros, D. G., Silburn, P., & Hall, B. (2005). Beyond verbal fluency: investigating the long-term effects of bilateral subthalamic (STN) deep brain stimulation (DBS) on language function in two cases. Neurocase, 11(2), 93-102. ↩
- de Jong, E., van Dijk, M., Oterdoom, M., & Rofes, A. (2023). The ability of thematic and taxonomic semantic categorization in people with Essential Tremor & Parkinson’s Disease, pre- and post-DBS surgery. Tabu Dag (43rd edition). ↩