Rejuvenating senescence of neural stem cells
Rejuvenating senescence of neural stem cells
Author: José R. Pineda got his Ph.D. from University of Barcelona in 2006. Since 2007 he has worked for Institut Curie and The French Alternative Energies and Atomic Energy Commission. Currently he is a researcher of the UPV/EHU. He investigates the role of stem cells in physiologic and pathologic conditions.
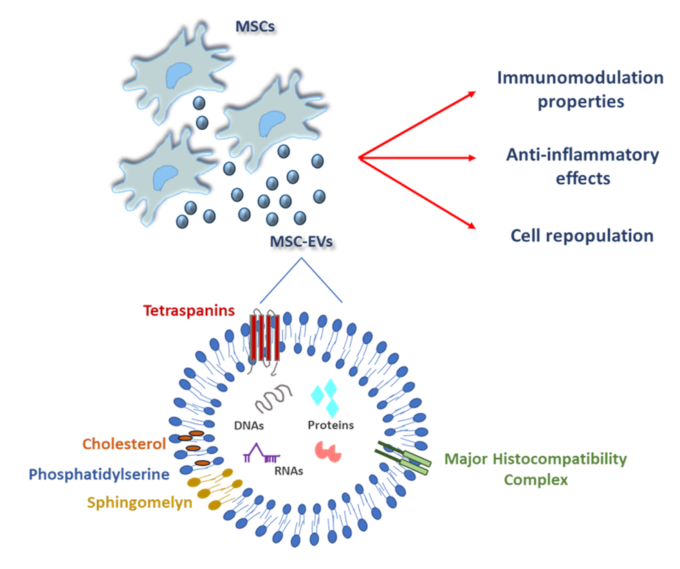
The research group of Wang in a recent paper explored how neural stem cells (NSCs) age under ischemic conditions and tested whether treatment with induced mesenchymal stem cell-derived small extracellular vesicles (iMSC-sEVs) could alleviate this aging process to aid recovery after ischemic stroke 1. Extracellular vesicles (EVs) are emerging as key players that act as tiny packages that cells send out to interact with nearby or distant cells. This form of “cellular mail” is used by virtually all cell types. Recent research has shed light on the molecular composition of EVs produced by mesenchymal stem cells, revealing promising avenues for clinical applications 2 (Figure 1).
The ischemic stroke is a condition caused by a blockage in blood vessels, leading to a lack of oxygen and glucose to the brain, damaging neural tissues. On the other part, neural stem cells (NSCs) are immature cells resident in neurogenic niches, able to differentiate into glia and neurons. It has been found that these cells are able to activate and give rise to new neurons after ischemic damage 3. However, under these stimuli they undergo a gradual process in which progressively they lose their ability to divide and function properly, a process known as cellular senescence.
Liu and collaborators used an in vitro oxygen-glucose deprivation (OGD) model to simulate ischemic conditions in cell cultures. They isolated NSCs from neonatal mice, and after cell expansion and subculture, they divided clonal flasks into parallel conditions varying the duration (1h, 2h and 9h) of the OGD stimulus to do a transcriptomic sequencing analysis (a genetic technique used to analyze gene expression). By this approach, they could determine the intracellular changes over time due to OGD. Following the results, they identified changes in eight groups of genes, especially those related to a decline in activity and neuronal differentiation. Indeed, a detailed analysis of genes activated after 9 hours of OGD revealed markers of cellular senescence, such as PCNA-p21 complex and oxidative stress response and p53 protein, suggesting that prolonged OGD induced NSC aging through oxidative stress and probably indirect DNA damage.
Next, to identify the correlation between NSC senescence and decreased proliferative and differential capacities after stroke, they used a mouse model of MCAO (“Middle Cerebral Artery Occlusion” is a surgical technique to simulate ischemic stroke). Using laser speckle imaging system, they were able to determine the regional cerebral blood flow around the cortical infarct during ischemia–reperfusion and determine the decrease of blood flow around the infarcted area. Interestingly, using the combination of Sox2 and GFAP immunostaining they also found a significant reduction in NSCs cell population. Having observed the reduced numbers of stem cells, they decided to analyze their progeny, that is, the immature neuronal progenitors using the cellular marker doublecortin (DCX). They also found reduced numbers of these neuronal progenitors in the dentate gyrus of the hippocampus (a brain region critical for short-term memory cognitive functions) post-stroke. Interestingly, they also found an increase of p16INK4a (a tumor supresor protein known to drastically increase during cell ageing) in the co-stained Sox2 (NSCs) cell population.
These results were in agreement with the observed p21 increase in vitro, suggesting that oxygen deprivation also accelerated NSCs aging process in vivo. Thus, to protect this cellular decline, the authors designed an experimental approach to treat NSCs with small extracellular vesicles of MSC derived from iPSC (iMSC-sEVs). With this strategy they could 1) to asses the reversibility or cellular protection of the senescence determined 9 hours after of OGD and 2) to validate the reprogramming towards mesenchymal stem cells of a population of induced pluripotent stem cells as a therapeutic tool.
iMSC-sEVs were isolated by seriated centrifugations and a final ultracentrifugation of 100.000 rpm during 70min (to have a reference of the speed of rotation a car engine or a washing machine barely reach 5.000 rpm or 1.400 rpm respectively. Thanks to the enormous centrifugal force generated by this strategy, ultra-minuscule fractions of biological material of very low weight can be sedimented and collected). These vesicles had a size range from 60 to 120 nm (120 nm are 0,00012 millimetres) and carried markers as CD9, CD63, and TSG101 characteristic of exosomal fraction. Treatment with iMSC-sEVs mitigated signs of cellular aging in NSCs, evidenced by reduced levels of aging markers and improved cell proliferation. Next step was to test if this strategy could have potential beneficial effects in vivo.
After treatment with iMSC-sEVs, there found was a noticeable enhancement in the number and regenerative capacity of hippocampal NSCs in MCAO mice. Synaptic proteins such as PSD95, Gap43, Syn1 showed increased levels, indicating an improved synaptic function. Furthermore, behavioral tests of iMSC-sEVs-treated MCAO animals demonstrated improved spatial memory and cognitive abilities compared to untreated ones.
In conclusion, they found that 1) senescence of NSCs induced by OGD and stroke accelerating its cellular aging, which hampers their ability to produce new neurons and neuronal circuits. 2) Treatment with iMSC-sEVs can reverse some aging effects on NSCs, improving their regenerative capacity and contributing to improved NSC function and synaptic repair and better recovery after ischemic stroke, unraveling the therapeutic potential of iMSC-sEVs.
References
- Liu, Jiayuan, Li Peng, Lingwei He, Tianyue Yin, Yuhao Du, Mengmeng Yang, Ping Wu, et al. (2024) “Induced Mesenchymal Stem Cells-Small Extracellular Vesicles Alleviate Post-Stroke Cognitive Impairment by Rejuvenating Senescence of Neural Stem Cells.” Journal of Molecular Neuroscience 74, 1: 29. https://doi.org/10.1007/s12031-024-02191-w. ↩
- Alberti, G.; Russo, E.; Corrao, S.; Anzalone, R.; Kruzliak, P.; Miceli, V.; Conaldi, P.G.; Di Gaudio, F.; La Rocca, G. (2022) “Current Perspectives on Adult Mesenchymal Stromal Cell-Derived Extracellular Vesicles: Biological Features and Clinical Indications. “Biomedicines, 10, 2822. https://doi.org/10.3390/biomedicines10112822 ↩
- Okano H, Sakaguchi M, Ohki K, Suzuki N, Sawamoto K. (2007) “Regeneration of the central nervous system using endogenous repair mechanisms.” J Neurochem. Sep;102(5):1459-1465. https://doi.org/10.1111/j.1471-4159.2007.04674.x ↩