Depletion of dendritic cells in established tumors suppresses immunotherapy efficacy
Depletion of dendritic cells in established tumors suppresses immunotherapy efficacy
The ability to uptake cellular debris and process the engulfed antigens for MHC class I presentation is mainly performed by a minority subset of dendritic cells 1. Immunologists identified them as “conventional-type 1 dendritic cells”, and characterized them identifying the presence of surface coexpression of CD11c, XCR1, and DNGR-1 molecules 2. In mice, two subsets of cDC1 cells have been identified depending on expression of the CD103 integrin, which defines the CD103+ migratory cDC1s and a CD103− resident subtype, constitutively located in secondary lymphoid organs 3. Much of the research pertaining to cDC1 function comes from the fact that this subset of DC needs the transcription factors BATF3 and IRF8 for their ontogeny in the bone marrow 4. It has been previously shown that tumor immunotherapy with anti–PD-1 and anti-CD137 mAbs (which act by boosting antitumor immune responses) was ineffective in cDC1-deficient Batf3−/− mice 5.
In humans, cDC1 cells can be identified by their shared expression of XCR1 and DNGR-1, similar to their mouse counterparts 67. The role of cDC1 cells in cancer immunology and immunotherapy was highlighted by findings showing that tumors which do not produce the chemokines CCL4 or CCL5, which attract cDC1 cells, tend to be poorly immunogenic 8. In human tumors, cDC1-specific transcriptional signature and Batf3 expression is strongly associated with high levels of CD8+ T-cell infiltration 9.
One important question that remains unanswered is whether cDC1 cells are required once immunotherapy has been instigated to mediate and sustain the beneficial effect of treatment. With the help of XCR1-DTR-Venus transgenic mice 10, the authors of the present work 11 have demonstrated that diphtheria toxin (DT)–mediated depletion of cDC1 cells upon treatment hampers response to various immunotherapy agents. Moreover, microscopy imaging indicates that cDC1 cells actively interact with tumor antigen-specific T cells in the tumor microenvironment and are involved in tumor T-cell infiltration.
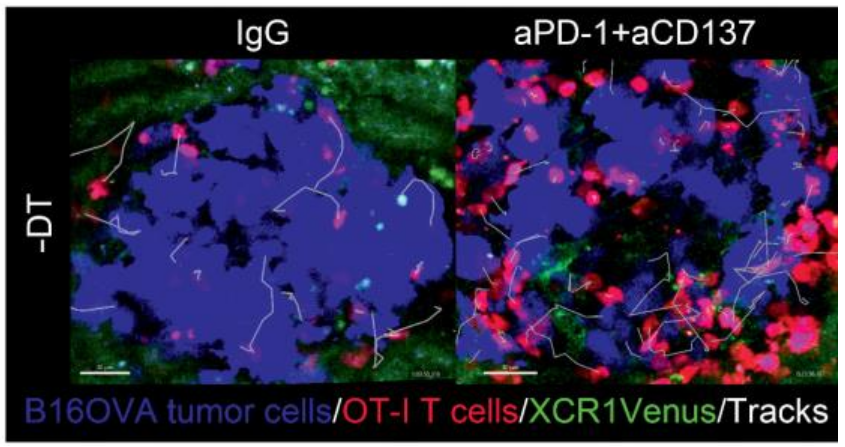
Modified from Teijeira A et al. (2022) Cancer Res. doi: 10.1158/0008-5472.CAN-22-1046.
Firstly, to investigate the need for the presence of cDC1 cells during immunotherapy, they used XCR1-DTR-Venus transgenic mice (10) to engraft MC38-derived tumors (a commonly used murine model for colorectal carcinoma) either subcutaneousor intraperitoneally DT injection. Twenty-four hours after DT treatment, DCs almost disappeared and, in excised tumors, all Venus+ DCs were absent as a result of DT treatment. Then, they used the MC38 tumor model to characterize the need for cDC1 cells during treatment with efficacious immunotherapies. MC38-derived subcutaneous tumors partially responded to anti-CD137, or to anti–PD-1 mAbs and were strongly improved when a combination of anti-CD137 plus anti–PD-1 mAbs was used (5). However, the partial efficacy of treatment of anti–PD-1 or anti-CD137 mAbs and the synergistic immunotherapy were completely lost in DT-treated mice. Focusing on the efficacy of anti–PD-1+anti-CD137 antibodies, they used multiplex tissue immunofluorescence to study the lymphocyte infiltrates of MC38 tumors under therapy with and without cDC1 depletion. Interestingly, depletion of cDC1 cells during immunotherapy with immunostimulatory mAbs reduced the tumor infiltration by T cells. Interestingly, cells remained on the rim of the invasive tumor margins without penetrating to the center of the engrafted tumor nodules.
It has been recently reported that during chronic lymphocytic choriomeningitis (LCMV) infection cDC1 are able to support TCF1+ CD8+ precursor cells 12. To test the functional effects of cDC1 absence during immunotherapy they used the B16–OVA model for cancer immunotherapy, which expresses ovalbumin (OVA) in order to facilitate strong immune responses to tumor antigens. Thus, they implanted B16.OVA tumors in XCR-1 DTR mice and showed that immunotherapy enhanced the overall number of proliferating CD8+ T-cells, despite these cells were TCF1low, mainly when cDC1 were absent. The autors confirmed that inside the tumor compartment the CD8+ T-cells had an impaired infiltration as a result of cDC1 depletion. To test whether these changes in CD8 T-cells were observable in another tumor model, they analyzed endogenous CD8+ T-cells infiltrating MC38 tumors in the same experimental conditions. Likewise, they observed the almost complete loss of TCF1 expression and enhanced proliferation of the TCF1low populatio upon cDC1 depletion.
The selective expression of Venus in XCR1+ cDC1 cells in XCR1-DTR-Venus mice offered an opportunity for in vivo time-lapse confocal microscopy to trace their presence and behavior. To performd this approach, they used a B16.OVA melanoma variant transfected to express a fluorescent protein in which mice bear metastatic melanoma lesions in the liver. Focusing on cDC1 cells, they were able to trace their behavior and their interactions with CD8+ T-cells. When the experiments were performed in mice receiving anti–PD-1 plus anti-CD137 mAbs, more cDC1 cells could be found in the metastatic foci and a fraction of cDC1 cells. Furthermore, in this condition, cells not only established more durable interactions with CD8+ T-cells but also experimented an enhanced speed with increased directional movement. Next, they examined T-cell behavior by comparing mice in which cDC1 cells had been depleted using DT, and/or treated with immunotherapy involving anti–PD-1 plus anti-CD137 mAbs. Notably, under immunotherapy, CD8+ T-cells readily infiltrated the tumor. However, this infiltration was significantly reduced when cDC1 cells were depleted by DT.
Overall, this work indicates that cDC1 cells are not only important to mediate infiltration of T cells recognizing tumor antigens, but also are able to change the functional T-cell behavior in the tumor tissue microenvironment.
Author: Marta Irigoyen is a postdoctoral researcher at CIC bioGUNE
References
- Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012; 12: 557-569. PMID: 22790179 DOI: 10.1038/nri3254. ↩
- Cabeza-Cabrerizo M, Cardoso A, Minutti CM, Pereira da Costa M, Reis e Sousa C. Dendritic Cells Revisited. Annu Rev Immunol. 2021; 39: 131-166. PMID: 33481643 DOI: 10.1146/annurev-immunol-061020-053707. ↩
- Edelson BT, KC W, Juang R, Kohyama M, Benoit LA, Klekotka PA, Moon C, Albring JC et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med. 2010; 207: 823-836. PMID: 20351058 DOI: 10.1084/jem.20091627. ↩
- Murphy TL, Grajales-Reyes GE, Wu X, Tussiwand R, Briseño CG, Iwata A, Kretzer NM, Durai V et al. Transcriptional Control of Dendritic Cell Development. Annu Rev Immunol. 2016; 34: 93-119. PMID: 26735697 DOI: 10.1146/annurev-immunol-032713-120204. ↩
- Sánchez-Paulete AR, Cueto FJ, Martínez-López M, Labiano S, Morales-Kastresana A, Rodríguez-Ruiz ME, Jure-Kunkel M et al. Cancer Immunotherapy with Immunomodulatory Anti-CD137 and Anti-PD-1 Monoclonal Antibodies Requires BATF3-Dependent Dendritic Cells. Cancer Discov. 2016; 6: 71-9. PMID: 26493961 DOI: 10.1158/2159-8290.CD-15-0510. ↩
- Poulin LF, Reyal Y, Uronen-Hansson H, Schraml BU, Sancho D, Murphy KM, Håkansson UK, Moita LF et al. DNGR-1 is a specific and universal marker of mouse and human Batf3-dependent dendritic cells in lymphoid and nonlymphoid tissues. Blood. 2012; 119: 6052-626. PMID: 22442345 DOI: 10.1182/blood-2012-01-406967. ↩
- Bachem A, Güttler S, Hartung E, Ebstein F, Schaefer M, Tannert A, Salama A, Movassaghi K et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med. 2010; 207: 1273-1281. PMID: 20479115 DOI: 10.1084/jem.20100348. ↩
- Ruiz de Galarreta M, Bresnahan E, Molina-Sánchez P, Lindblad KE, Maier B, Sia D, Puigvehi M, Miguela V et al. β-Catenin Activation Promotes Immune Escape and Resistance to Anti-PD-1 Therapy in Hepatocellular Carcinoma. Cancer Discov. 2019; 9: 1124-1141. PMID: 31186238 DOI: 10.1158/2159-8290.CD-19-0074. ↩
- Spranger S, Luke JJ, Bao R, Zha Y, Hernandez KM, Li Y, Gajewski AP, Andrade J et al. Density of immunogenic antigens does not explain the presence or absence of the T-cell-inflamed tumor microenvironment in melanoma. Proc Natl Acad Sci U S A. 2016; 113: E7759-E7768. PMID: 27837020 DOI: 10.1073/pnas.1609376113. ↩
- Yamazaki C, Sugiyama M, Ohta T, Hemmi H, Hamada E, Sasaki I, Fukuda Y, Yano T et al. Critical roles of a dendritic cell subset expressing a chemokine receptor, XCR1. J Immunol. 2013; 190: 6071-6082. PMID: 23670193 DOI: 10.4049/jimmunol.1202798. ↩
- Teijeira A, Garasa S, Luri-Rey C, de Andrea C, Gato M, Molina C, Kaisho T, Cirella A et al. Depletion of Conventional Type-1 Dendritic Cells in Established Tumors Suppresses Immunotherapy Efficacy. Cancer Res. 2022; 82: 4373-4385. PMID: 36130020 DOI: 10.1158/0008-5472.CAN-22-1046. ↩
- Dähling S, Mansilla AM, Knöpper K, Grafen A, Utzschneider DT, Ugur M, Whitney PG, Bachem A, et al. Type 1 conventional dendritic cells maintain and guide the differentiation of precursors of exhausted T cells in distinct cellular niches. Immunity. 2022; 55: 656-670.e8. PMID: 35366396 DOI: 10.1016/j.immuni.2022.03.006. ↩