Biomarkers of chemoresistant leukemic cells in human T-ALL
Biomarkers of chemoresistant leukemic cells in human T-ALL
Author: Marta Irigoyen is a postdoctoral researcher at CIC bioGUNE
T-cell acute lymphoblastic leukemia (T-ALL) is characterized by the accumulation of genetic lesions that induce differentiation arrest, survival and aberrant proliferation of immature T-cell progenitors 1. Although T-ALL prognosis has significantly improved due to intensive chemotherapy, relapses still occur in 20% of pediatric patients and 50% of adult patients, often with a dismal outcome2. To accurately classify patients based on risk of relapse, it is imperative to identify biomarkers indicative of relapse. These biomarkers can then be leveraged to identify novel drug targets and develop alternative therapies to treat patients with chemoresistant T-ALL.
Leukemic cells infiltrate various tissues including the bone marrow (BM), which provides an important microenvironment for T-ALL expansion. In the BM, leukemic cells take advantage of several BM-secreted factors that support the initiation or spread of acute myeloid leukemia or acute lymphoid leukemia 3. In addition to these soluble factors, multiple cells within the BM niches also interact with leukemic cells4. Among these, adipocytes, which constitute BM adipose tissue (BMAT), are rare in young bones but markedly increase during the aging process or radio/chemotherapy5. Due to these direct effects, understanding the interactions between adipocytes and leukemic cells may thus provide insight for improved patient care. Indeed, has been previously shown that ALL cells infiltrate BMAT-rich sites, in which they display decreased metabolism and translational processes, accumulation in the G0 cell cycle phase, and a chemoresistance phenotype (5). These characteristics may be leveraged to develop effective therapeutic alternatives to overcome BMAT-induced chemoresistance. Given the role that the BM microenvironment plays in T-ALL chemoresistance, Calvo J. and coworkers aimed to fully characterize the chemoresistant quiescent T-ALL cells from BMAT sites 6.
To characterize the leukemic cell diversity at different BMAT-poor/rich sites (5), authors isolated cells from BMAT-poor BM (referred as red-BM) (femur, thoracic vertebrae) and BMAT-rich BM (referred as yellow-BM) (tail vertebrae), and then purified human leukemic cells for scRNAseq analysis (a technology that enables the dissection of gene expression at single-cell resolution). The utilization of the principal component analysis enabled the visualization of eight different clusters of leukemic cells. While all clusters exhibited an equal distribution of cells across BM regions, Cluster 4 comprised 91.2% of cells from BMAT-rich sites. Analysis of the cell cycle status in the scRNAseq dataset showed that cells from Clusters 0 and 4 were predominantly in the G1 phase, whereas cells from the other clusters were in the S/G2M phases. Low mRNA counts used to distinguish between the G0 and G1 phases as well as the absence of MKI67 expression indicated that Cluster 4 contained cells primarily in G0 phase. Using flow cytometry analysis, they found that leukemic cells from the femur and thorax/BMAT-poor BM were activated and cycling cells, while cells from tail vertebrae/BMAT-rich BM were mostly in a G0/quiescent state. Collectively, these findings underscore significant leukemic cell diversity in both red and yellow BM sites. The single-cell transcriptomic analysis further indicated that most leukemic cells isolated from the BMAT-rich/ yellow BM sites constitute a homogeneous cluster associated with quiescence.
The transcriptomic signature of Cluster 4 was further compared with the label-retaining cell (LRC) transcriptomic signature that defines quiescent B-ALL cells and is enriched in B-ALL minimal residual disease (MRD) 7. Thus, they observed a significant enrichment of the LRC signature in cells from Cluster 4, which underscores a shared transcriptomic signature in quiescent and chemoresistant T/B-ALL cells. To define the biological mechanisms that are associated with quiescent/resistant leukemic cells, they performed a Gene Set Enrichment Analysis (GSEA) of the upregulated genes from Cluster 4. Consistent with previous observations (5), most of the basal cellular processes such as “protein synthesis, mRNA catabolic processes and metabolism” were downregulated in leukemic cells from Cluster 4. The only significantly upregulated Gene Ontology (GO) biological processes highlighted in these resting leukemic cells were “Cell Adhesion” and “Biological Adhesion”. Among the top five enriched genes involved in “Cell Adhesion”, they found CD44, which is a ubiquitous cell surface glycoprotein, implicated in solid cancer and leukemia migration/adhesion 8. Then, they determined which CD44 isoform was expressed in T-ALL cells and found that leukemic cells carry the CD44 standard isoform, which is expressed at higher levels in cells from BMAT-rich/tail vertebrae compared with BMAT-poor BM. Furthermore, surface CD44 protein expression was also higher in leukemic cells from BMAT-rich/tail vertebrae, as confirmed by western blot analysis, suggesting that high CD44 expression is associated with leukemic quiescence.
To investigate whether the quiescent CD44 high T-ALL cells are chemoresistant, they administered a chemotherapy treatment in mice transplanted with human T-ALL cells 9. Thus, chemotherapy treatment reduced tumor burden in the spleen and peripheral blood, and the reduction in T-ALL cell burden was more pronounced in BMAT-poor BM than in BMAT-rich BM. Next, they characterized the residual BM T-ALL cells that resisted chemotherapy. The analysis revealed 2 clusters of a total of 12, in which CD44 high leukemic cells were predominantly localized. Besides, the proportion of these clusters was approximately 50% in BMAT-rich sites and <3% in BMAT-poor sites for placebo-treated mice. To further characterize the selected leukemic cells post-chemotheraphy treatment, they analyzed CD44 expression in leukemic cells from treated or untreated femurs from mice xenografted with human T-ALL. As expected, leukemic cells from chemotheraphy-treated femurs displayed elevated CD44 transcripts and protein expression levels and were primarily quiescent. To explore the benefit of high CD44 expression as a prognosis biomarker, author’s evaluated CD44 expression levels in a patient cohort which enabled the analysis of the expression profiles of 264 diagnostic human T-ALL samples 10. The results revealed that patients with high CD44 mRNA expression were significantly associated with several oncogenic subgroups, which present a poor prognosis after chemotherapy 11.
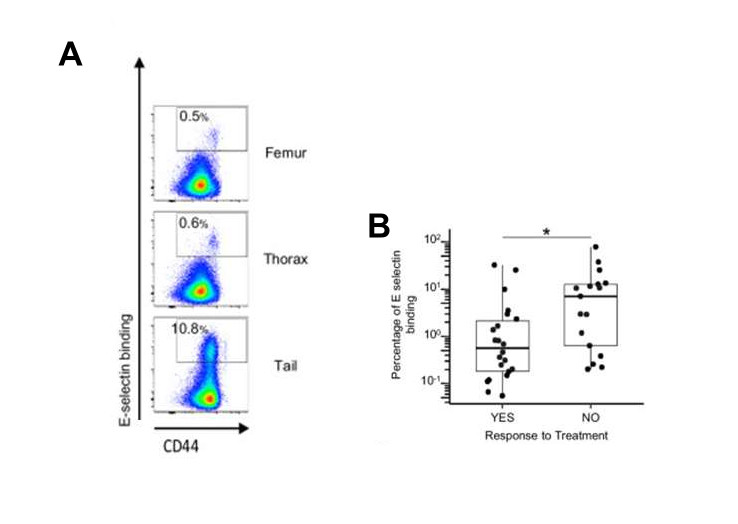
CD44 is known to interact with a wide variety of ligands, such as hyaluronic acid and E-selectin 12. To demonstrate E-selectin binding activity, CD44 must bear multiple sialyl Lewis X (sLeX) motifs as a terminal structure. Upon analysis of the sLex motif on the surface of leukemic cells, they found that leukemic cells expressing high levels of CD44 from BMAT-rich and BMAT-poor sites harbored sLex motifs. Subsequently, they evaluated the attachment efficacy of a chimeric soluble human E-selectin-IgG1 fusion protein to leukemic cells recovered from various BM regions. As anticipated, a greater proportion of leukemic cells from the BMAT-rich site bound E-selectin compared with the BMAT-poor sites. Using flow cytometry analysis, they found that binding to E-selectin was detected in cells with the highest levels of CD44 expression. E-selectin binding was also measured in leukemic cells from 46 patient samples. Using the clinical information available, they classified the samples into two groups: sensitive and aggressive cases, the latter including refractory and relapsed patients. Assessing information regarding patient treatment response and E-selectin binding levels revealed that relapse/refractory patients (the “NO” response group) had 10-fold higher levels of E-selectin binding compared with chemotherapy-sensitive patients (the “YES” response group). Interestingly, the T-ALL cells more prone to E-selectin binding were CD44high.
In summary, authors found that T-ALL cells capable of E-selectin binding are CD44 high both in T-ALL models and human patients. From a clinical perspective, their results suggest that high CD44 and E-selectin binding potential can be leveraged to stratify relapse/refractory patients.
References
- Belver L, Ferrando A. The genetics and mechanisms of T cell acute lymphoblastic leukaemia. Nat Rev Cancer. 2016;25;16:494-507. doi: 10.1038/nrc.2016.63 PMID: 27451956. ↩
- Vadillo E, Dorantes-Acosta E, Pelayo R, Schnoor M. T cell acute lymphoblastic leukemia (T-ALL): New insights into the cellular origins and infiltration mechanisms common and unique among hematologic malignancies. Blood Rev. 2018;32:36-51. doi: 10.1016/j.blre.2017.08.006 PMID: 28830639. ↩
- Calvo J, Fahy L, Uzan B, Pflumio F. Desperately seeking a home marrow niche for T-cell acute lymphoblastic leukaemia. Adv Biol Regul. 2019;74:100640. doi: 10.1016/j.jbior.2019.100640. PMID: 31378700. ↩
- Schepers K, Campbell TB, Passegué E. Normal and leukemic stem cell niches: insights and therapeutic opportunities. Cell Stem Cell. 2015;16:254-67. doi: 10.1016/j.stem.2015.02.014. PMID: 25748932. ↩
- Heydt Q, Xintaropoulou C, Clear A, Austin M, Pislariu I, Miraki-Moud F, Cutillas P, Korfi K et al. Adipocytes disrupt the translational programme of acute lymphoblastic leukaemia to favour tumour survival and persistence. Nat Commun. 2021;12:5507. doi: 10.1038/s41467-021-25540-4. PMID: 34535653. ↩
- Calvo J, Naguibneva I, Kypraios A, Gilain F, Uzan B, Gaillard B, Bellenger L, Renou L et al. High CD44 expression and enhanced E-selectin binding identified as biomarkers of chemoresistant leukemic cells in human T-ALL. Leukemia. 2025;39:323-336. doi: 10.1038/s41375-024-02473-7. PMID: 39580584. ↩
- Ebinger S, Özdemir EZ, Ziegenhain C, Tiedt S, Castro Alves C, Grunert M, Dworzak M, Lutz C et al. Characterization of Rare, Dormant, and Therapy-Resistant Cells in Acute Lymphoblastic Leukemia. Cancer Cell. 2016;30:849-862. doi: 10.1016/j.ccell.2016.11.002. PMID: 27916615. ↩
- Krause DS, Lazarides K, von Andrian UH, Van Etten RA. Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat Med. 2006;12:1175-80. doi: 10.1038/nm1489. PMID: 16998483. ↩
- Szymanska B, Wilczynska-Kalak U, Kang MH, Liem NL, Carol H, Boehm I, Groepper D, Reynolds CP et al. Pharmacokinetic modeling of an induction regimen for in vivo combined testing of novel drugs against pediatric acute lymphoblastic leukemia xenografts. PLoS One. 2012;7:e33894. doi: 10.1371/journal.pone.0033894. PMID: 22479469. ↩
- Liu Y, Easton J, Shao Y, Maciaszek J, Wang Z, Wilkinson MR, McCastlain K, Edmonson M et al. The genomic landscape of pediatric and young adult T-lineage acute lymphoblastic leukemia. Nat Genet. 2017;49:1211-1218. doi: 10.1038/ng.3909. PMID: 28671688 ↩
- Coustan-Smith E, Mullighan CG, Onciu M, Behm FG, Raimondi SC, Pei D, Cheng C, Su X et al. Early T-cell precursor leukaemia: a subtype of very high-risk acute lymphoblastic leukaemia. Lancet Oncol. 2009;10:147-56. doi: 10.1016/S1470-2045(08)70314-0. PMID: 19147408. ↩
- Zöller M. CD44, Hyaluronan, the Hematopoietic Stem Cell, and Leukemia-Initiating Cells. Front Immunol. 2015;6:235. doi: 10.3389/fimmu.2015.00235. PMID: 26074915. ↩