Vascular permeability in the bone marrow and drug response in Acute Myeloid Leukemia
Vascular permeability in the bone marrow and drug response in Acute Myeloid Leukemia
Author: Marta Irigoyen is a postdoctoral researcher at CIC bioGUNE
Acute myeloid leukemia (AML) is a hematological malignancy arising from the occurrence of genetic mutations in hematopoietic progenitors, which cause a blockage in the maturation and an uncontrolled growth of leukemic blasts in the bone marrow (BM). The resistance to current therapies and relapse remain a major clinical challenge. For this reason, a novel approach is required to identify and target common features within this complex disease. For example, the BM microenvironment (which is the site where leukemic cells arise) progressively adapts to the malign cells expanding their influence, and eventually culminating in resistance to therapy 1. In fact, recent findings indicate that myeloid malignancies also affect the function of the BM niche, pointing to the existence of an active bidirectional crosstalk between leukemic cells and the microenvironment 2. Similar to what has been observed in solid cancers, AML has been associated with an increase in microvascular density (MVD) and production of pro-angiogenic factors, notably vascular endothelial growth factor (VEGF)3. However, clinical trials incorporating anti-VEGF inhibitors have not produced encouraging results 4, suggesting that targeting pro-angiogenic cytokines may not be the best strategy for disrupting the crosstalk between AML and the vascular niche. Thus, Passaro D. and coworkers aim to study the in vivo picture of the abnormalities associated with the BM vasculature induced by AML engraftment, which could represent potential targets in AML 5.
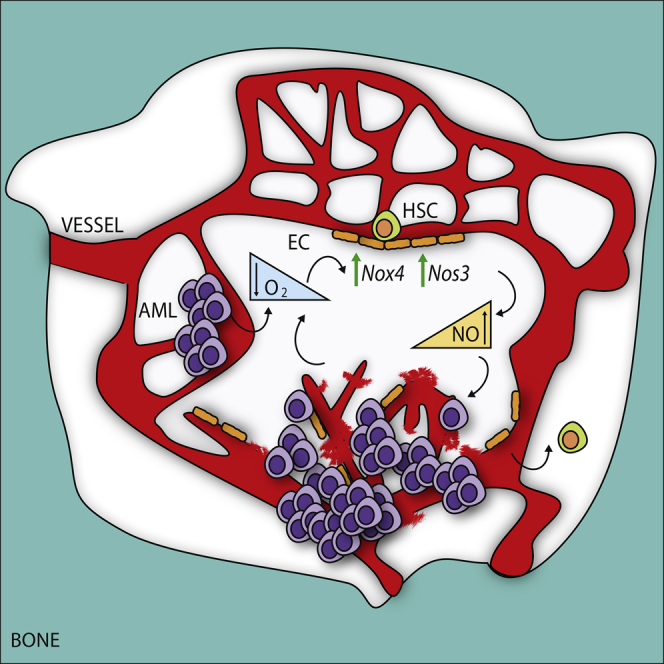
To provide a detailed picture of the BM vasculature in AML, the authors studied the status of the vascular niche in human AML patient-derived xenografts (PDX). Interestingly, they observed an expansion of the endothelial compartment among the non-hematopoietic stroma. Thus, the percentage of endothelial cells (ECs) was positively correlated to the leukemic engraftment. Next, they analyzed the architecture of the BM vasculature and they found some interesting abnormalities: First, they found a loss of the regularity of sinusoidal structures and second, they observed a reduction of the diameter of vessels in human AML xenografts. Regarding BM perfusion, they observed the presence of many poorly perfused areas in the BM of AML xenografts. Thus, they aimed to test whether AML engraftment also affected BM oxygenation and observed that human AML engraftment increased hypoxia homogeneously throughout the bones and most of the vessels were close to these hypoxic areas. Besides, when they studied the vascular barrier function, they observed an important leakage of the vasculature in the BM of mice engrafted with human AML cell lines compared with control non-transplanted mice.
Since it is still debated whether AML-derived angiogenesis is reduced in patients upon chemotherapy 6, they aimed to analyze the levels of VEGF in BM biopsies. They found an increase in VEGF in the majority of patients’ post-treatment samples, which suggests a persistent pro-angiogenic environment after chemotherapy. Moreover, they focused the study on the analysis of the state of the vascular niche in AML PDX after chemotherapy. To do that, they used a protocol of preclinical treatment observing that residual AML cells localized in close proximity to the vasculature after treatment. Interestingly, despite the leukemic engraftment being significantly reduced, the vascular permeability was still very high, even similar to mice treated with the control solvent. Finally, the number of ECs was not altered in AML PDX after treatment, the BM hypoxia was not normalized upon removal of leukemic cells and most of the vessels were still close to hypoxic areas upon treatment.
To identify common downstream effectors responsible of the angiogenic stimulation in the vascular niche, authors studied the transcriptome of BM-derived ECs retrieved from mice engrafted with different AML patient-derived cells and compared them with normal ECs. Interestingly, they identified a common deregulated signature associated with human AML engraftment, directly related to an abnormal vasculature. For example, the expression of adhesion molecules changed upon AML engraftment, with upregulation of integrins (adhesion molecules involved in migration, growth, and survival of newly formed vessels). On the contrary, they also noticed reduced expression of tight junction components (molecules responsible for maintaining endothelial layer integrity). Besides, among the common highest upregulated genes they found Nox4, a NAPDH oxidase expressed in the vasculature and involved in the response to hypoxia via production of reactive oxygen species (ROS), activation of nitric oxide synthase 3 (NOS3), and the release of nitric oxide (NO) 7. Since NO is the major mediator of vascular permeability in both physiological and several pathological conditions 8, they next investigated whether the AML-derived upregulation of Nox4 in ECs was able to affect the NO production in the BM vascular niche, finding increased levels in AML xenografts. Accordingly to the present results, they also observed a significant increase in the NO levels in AML patient-derived BM biopsies. Thus, the upregulation of Nox4 in ECs was indicative of an overactivation of NOS3 becoming responsible for the observed NO levels in the BM. Next, authors analyzed the expression of NOS3 in the two major cellular components of the vascular niche: the endothelial and the mesenchymal perivascular cells. Interestingly, they observed that ECs represented the major compartment expressing NOS3 and that NOS3 protein expression and activation were significantly increased in ECs retrieved from mice engrafted with human AML patient-derived samples. In contrast, NOS3 was not increased in the mesenchymal compartment upon AML engraftment.
To test whether the exogenous production of NO had a role in leukemia transplantation and progression, they aimed to graft murine AML leukemic cells into Nos3-knockout (Nos3-KO) recipient mice and to characterize the status of the vasculature and the progression of the disease. Interestingly, the development of leukemia was significantly delayed in Nos3-KO mice observing a reduced leukemic engraftment in the BM and spleen (provoking a reduction of the disease penetrance). Moreover, Nos3 inactivation together with the concomitant chemotherapy treatment was able to reduce leukemic engraftment and the vascular leakiness associated with BM of AML-engrafted mice. To confirm these results, they used a PDX model allowing the AML cells to engraft into the animals before inhibiting the NO production (with NOS inhibitors). Accordingly to the previous data, the treatment of mice xenografted with NOS inhibitors in combination with chemotherapy significantly inhibited leukemic progression and reduced NOS3 activation, vascular leakiness, and BM hypoxia. Importantly, the combined treatment was more powerful than chemotherapy alone in reducing leukemic progression in the BM and the spleen and also significantly extended the “remission-like” phase of the disease compared with chemotherapy alone.
Since the increase in NO levels has been previously associated with an increase in HSPC motility and egression from the BM, leading to a lower repopulation capacity of the BM stem cell pool after injuries 9, authors speculated that increased NO and vascular leakiness in AML could affect normal HSC. Therefore, they tested whether normalization of the vascular niche with NOS inhibitors would be beneficial for normal HSC function. Hence, they treated mice engrafted with normal HSPCs with NOS inhibitors and analyzed the effect on the stem cell pool and observed a reduced HSPC egression to the blood, accompanied by a specific increase of HSPCs in the BM. Besides, NO inhibition not only increased the number of HSPCs in the BM but also their stem cell function. They next tested whether NO inhibition would be beneficial for residual normal HSPC to outcompete AML cells during the relapse process. To achieve this, they simultaneously grafted normal human HSPCs and AML patient-derived cells intravenously into mice 10 and monitored the normal versus malignant engraftment in the presence or absence of long-term NOS inhibitor. Interestingly, the analysis of the BM engraftment at the end of the treatment showed that NOS inhibition favored the normal over leukemic engraftment in the BM.
Altogether, these results provide strong evidence for the importance of the alteration of the vascular niche in AML progression and relapse, via increased NO production by ECs, and call for clinical trials incorporating NOS inhibitors to target the abnormal vascular niche and improve the treatment response.
References
- Boulais PE, Frenette PS (2025) Making sense of hematopoietic stem cell niches. Blood. doi: 10.1182/blood-2014-09-570192. ↩
- Krause DS, Fulzele K, Catic A, Sun CC, Dombkowski D, Hurley MP, Lezeau S, Attar E et al. (2013) Differential regulation of myeloid leukemias by the bone marrow microenvironment. Nat Med. doi: 10.1038/nm.3364 ↩
- Kampen KR, Ter Elst A, de Bont ES (2017) Vascular endothelial growth factor signaling in acute myeloid leukemia. Cell Mol Life Sci. doi: 10.1007/s00018-012-1085-3. ↩
- Giles FJ, Bellamy WT, Estrov Z, O’Brien SM, Verstovsek S, Ravandi F, Beran M, Bycott P et al. (2006) The anti-angiogenesis agent, AG-013736, has minimal activity in elderly patients with poor prognosis acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS). Leuk Res. doi: 10.1016/j.leukres.2005.10.024. ↩
- Passaro D, Di Tullio A, Abarrategi A, Rouault-Pierre K, Foster K, Ariza-McNaughton L, Montaner B, Chakravarty P et al. (2017) Increased Vascular Permeability in the Bone Marrow Microenvironment Contributes to Disease Progression and Drug Response in Acute Myeloid Leukemia Cancer Cell. doi: 10.1016/j.ccell.2017.08.001. ↩
- Aref S, El Sherbiny M, Goda T, Fouda M, Al Askalany H, Abdalla D. (2005) Soluble VEGF/sFLt1 ratio is an independent predictor of AML patient outcome. Hematology PubMed: 16019458. ↩
- Craige SM, Chen K, Pei Y, Li C, Huang X, Chen C, Shibata R, Sato K et al. (2011) NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation doi: 10.1161/CIRCULATIONAHA.111.030775. ↩
- Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG et al. (2001) Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc. Natl. Acad. Sci. USA. doi: 10.1073/pnas.041359198. ↩
- Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. (2003) Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. doi: 10.1038/nm948. ↩
- McIntosh BE, Brown ME, Duffin BM, Maufort JP, Vereide DT, Slukvin II, Thomson JA (2015) Nonirradiated NOD,B6.SCID Il2rgamma-/- Kit(W41/W41) (NBSGW) mice support multilineage engraftment of human hematopoietic cells. Stem Cell Rep doi: 10.1016/j.stemcr.2014.12.005. ↩