Grand Designs at the molecular scale: building custom protein crystals
Grand Designs at the molecular scale: building custom protein crystals
Author: Mantas Liutkus, Postdoctoral Researcher at the Biomolecular Nanotechnology Laboratory in CIC biomaGUNE.
Order on a molecular scale is difficult to control. The systems with highest possible order are crystals, formed by long arrays of repeating constituent components in all directions. The most familiar examples of crystals encountered in daily life are table salt and sucrose, the sugar in our kitchens. Every grain of table salt is a rigid lattice of sodium and chloride ions, held tightly in place by their charges. These crystals form spontaneously when a salt solution is allowed to evaporate, something most of us have seen with our own eyes in simple home experiments during primary school. Sucrose, on the other hand, is not made up of ions, but is instead a hydrated carbon chain (hence a carbohydrate). Nevertheless, this sugar also forms white crystalline cubes, with hydrogen bonds holding the sucrose molecules together.
Crystals of all shapes and sizes
As the molecular size of substances increases, it becomes increasingly more difficult to grow high quality crystals. Despite that, crystals of massive proteins are routinely grown in research laboratories in order to determine the structures of the proteins. The repeating structures diffract X-rays, with the diffraction patterns used to solve the protein structure.
The most familiar crystals to most people are three-dimensional crystals, such as the aforementioned salt or sugar crystals. However, two-dimensional crystals, usually in the form of thin sheets, or one-dimensional crystals are also common in nature. While 3D crystals are good for storage, locking the constituents in place, 2D and 1D crystals can have more diverse functions. Examples of two-dimensional crystals include the S-layer of bacterial envelopes or viral proteins preassembling into ordered surfaces during viral assembly, whereas actin and tubulin fibres are good representatives of 1D crystalline assemblies, keeping cells alive.
Crystals represent the most stable arrangement of substances. Thus, crystals of the same substance will always have the same crystal lattice, regardless of the size of the individual crystals. However, the kind of lattice a substance is prone to form is also generally not predictable, and the arrangement need to be determined experimentally. Thus, obtaining an ordered system with desired characteristics is a significant challenge.
Architects of protein crystals
A new study 1 developed a methodology for rational redesign of a crystal lattice of protein crystals into different crystal systems. The developed approach takes advantage of the sheer size of proteins.
Inside crystals, each protein makes multiple contacts, in the form of hydrogen bonds or ionic salt bridges, with neighbouring protein units. Due to the size and bulky nature of proteins, the contacts with each neighbour protein tend to be distributed on distinct surface regions. The interacting regions present viable engineering targets to control the protein-protein interactions, without affecting the overall shape or function of the protein. This is in stark contrast to mineral salts made of atoms that are symmetrical in all directions, or small molecules, where a small structural change can completely alter the chemical properties of the compound.
Winning by losing
The strategy followed primarily focussed on the surface topology of protein interactions. To grow into three-dimensional structures, each building block needs to make contacts with neighbouring blocks in all three spatial dimensions. If interactions in one of the directions is pre-empted, the system can only grow in the two remaining spatial dimensions, resulting in a two-dimensional system. Further control on the assembly can be imposed by adjusting the angles between the contact points. If the interaction sites are in a plane, then the building blocks would assemble into a flat 2D plane. An angle other than 180° between the contact points would introduce a curvature into the plane, forcing the plane to fold back onto itself to form a tube. If only one dimension for growth is allowed, then single fibres or arrays are forced to form.
The research tested the proposed methodology on crystals of tetratricopeptide repeat proteins. These helical proteins form long filaments that pack inside the crystal lattice as pipes while traversing the crystals, making identification of interaction sites very intuitive. Furthermore, the key contacts between the proteins are mediated by metal ions. Thus, interactions can be controlled by simply eliminating the metal-coordinating residues. To achieve the desired structural assemblies, the tetratricopeptide repeat proteins were reengineered to only coordinate metal ions on selected surfaces, and crystals based on these proteins were grown.
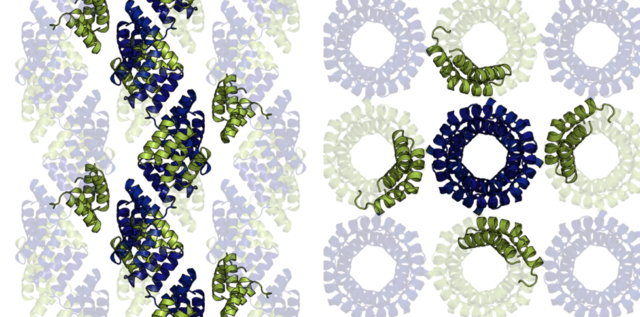
In line with the designs, proteins that had the metal coordination sites confined to a single plane crystallised to form planar, two-dimensional crystals. Proteins with metal-binding sites placed at an angle formed single-walled tubes with a self-folded crystalline surface. Therefore, by redesigning the crystal contacts, the three-dimensional crystal lattice was transformed into a 2D lattice, single-walled nanotubes, thin molecular fibres, or puckered planes.

In conclusion, tetratricopeptide repeat proteins were used as models, in part for their incredible stability, but also, as the name suggests, the repetitive nature of their structure. As the proteins are made up of repeating sequences, all metal coordination sites are identical, raising the possibility of unintended interactions during the redesign process. Nevertheless, the redesign strategy was successful, with all intended redesigned lattices achieved. This suggests that the crystal contact redesign strategy could be applied universally to most protein crystal lattices, opening the door for rapid generation of protein-based crystalline geometries.
The BRTA is a consortium that remains a step ahead of future socio-economic challenges worldwide and in the Basque Autonomous Community; it addresses them through research and technological development, thus projecting itself internationally. The BRTA centres collaborate to generate knowledge and transfer it to Basque society and industry so as to make them more innovative and competitive. The BRTA is an alliance of 17 R&D centres and cooperative research centres with the support of the Basque Government, the SPRI and the Chartered Provincial Councils of Araba, Bizkaia and Gipuzkoa.
References
- Liutkus M, Rojas AL, Cortajarena AL. (2025) Rational Crystal Contact Engineering for Programmable Self-Assembled Protein Architectures. Angew Chem Int Ed doi: 10.1002/anie.202516635 ↩
