A generalized approach for NMR studies of lipid–protein interactions
Author: Francisco J. Blanco is an Ikerbasque Research Professor at CIC bioGUNE
Molecular interactions in cell membranes, particularly lipid-protein interactions in their hydrophobic core, are difficult to analyse and remain poorly characterised despite high relevance in physiological and pathological processes. Structural rearrangements in membranes and embedded proteins, and the way molecular interactions contribute to their function are unresolved questions in biophysical chemistry.
For such studies, membranes are often mimicked by different aqueous lipid dispersions that form subject to parameters like lipid type, acyl chain length, concentration, mixture and stoichiometry. Commonly, micelles (spherical aggregates formed by short-chain lipids above a critical micelle concentration) or bicelles (discoidal aggregates formed by mixing short- and long-chained lipids) solubilise and stabilise membrane proteins for crystallisation and solution state Nuclear Magnetic Resonance (NMR) analysis.
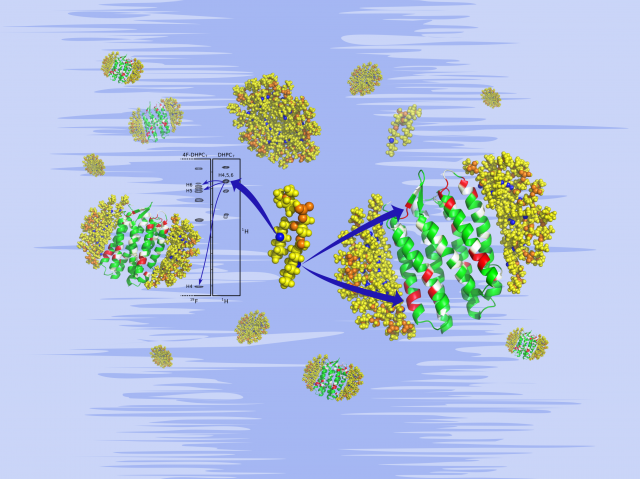
The known membrane protein structures, derived mainly by crystallography, provide little insight into lipid-protein interactions as bound lipid molecules are rarely visible. The conformational heterogeneity and molecular dynamics in lipid layers prevent their ordered crystallisation or modelling into electron microscopy structures. NMR spectroscopy, contrarily, does not require molecular order and lipid-protein contacts may be sampled at low resolution to gauge protein insertion into the lipid layer, e.g. by use of paramagnetic agents that perturb the NMR signals of nearby membrane protein atoms. Higher resolution analyses of lipid-protein contacts require more range-limited, directed, and unambiguous NMR indicators. Ultimately, specific lipid-protein contacts are revealed by intermolecular 1H,1H nuclear Overhauser effect signals. Yet, their identification and resolution are impeded by the poor 1H signal dispersion in lipid acyl chains. The classic workaround to correlate 1H with the better resolved 13C frequencies is impractical for lipids where the required 13C isotope enrichment is costly, may not resolve all overlap problems, and provides no distinct molecular marker from a likewise 13C labelled protein to single out inter- from intramolecular NOE signals.
Our new article 1 present the proof of concept of a generalized approach for NMR studies of lipid-protein interactions that relies on sparse fluorination of the lipid acyl chains and exploits fluorine both indirectly, as shift reagent affecting the NMR signals of nearby atoms, and directly, as 19F isotope with unique NMR properties.
The introduced fluorine atoms solve the NMR resolution problem for acyl chains by (i) increasing their 1H NMR signal dispersion via local deshielding, (ii) enabling clean molecular distinction via 19F filtering, and (iii) allowing further resolution enhancement via 19F editing. Orthogonal isotope filtering for 19F (on the acyl chain) vs. 13C or 15N (on the protein) will then yield NMR spectra with only intermolecular lipid-protein signals and well-dispersed acyl chain 1H frequencies. Fluorine may also perturb protein atoms close in space to indicate protein insertion into lipid layers similar to paramagnetic markers, but with minimal steric impact.
Replacing hydrogen by fluorine with its 20% larger atomic radius and higher electronegativity is a chemical modification with possible impact on the lipid’s biophysical properties. For instance, H/F substitution alters the charge distribution in the acyl chain, Also, interaction with membrane proteins may be affected As a drawback for NMR studies, H/F substitution reduces the 1H density and, thus, coverage of (fluoro)lipid-protein contacts via 1H,1H NOE signals. For all these reasons, the number of H/F substitutions in the lipid should be minimised.
In this introductory work, the focus was on the indirect NMR effects of fluorine and demonstrate its utility as both intra- and intermolecular shift reagent. A minimal fluorination scheme for acyl chains is derived, and it is shown that a single hydrogen-to-fluorine substitution induces a fully resolved 1H spectrum for 4F-DHPC7, a representative fluorolipid, without impairing micelle formation or stabilisation of the model membrane protein pSRII. The H/F substitution in the lipid provokes notable CSP for amide groups near both ends of the transmembrane seven-helix bundle indicating its asymmetric micelle insertion similar to paramagnetic markers.
In summary, the fully resolved 1H NMR spectrum of 4F-DHPC7 micelles validates the minimal fluorination scheme for lipid acyl chains and the utility of fluorine as intramolecular shift reagent to enhance their 1H signal dispersion. Importantly, the low fluorination level causes no deleterious impact on micelle formation, size, thermostability, and capacity to solubilise pSRII. TROSY spectra in DHPC7vs. 4F-DHPC7 micelles reveal small, but significant fluorine induced CSP for amide groups that cluster near both ends of the transmembrane seven-helix bundle and indicate asymmetric pSRII insertion into DHPC7 micelles, as derived in earlier studies with paramagnetic probes.
This agreement corroborates utility of fluorine also as intermolecular shift reagent to induce localised CSP in membrane proteins and gauge their insertion into the (fluoro)lipid layer. Other membrane proteins and sparsely fluorinated lipids or lipid-based systems may next be tested to establish the generality of the approach. The latter include, for instance, bicelles formed by mixtures of short- and long-chained lipids, or nanodiscs formed by lipid bilayers confined by a scaffold protein. These systems provide a more native-like membrane environment than micelles, but their larger size and asymmetry might cause faster 19F relaxation. While this can compromise 19F editing, 19F filtering may still be implemented with minimal sensitivity to relaxation. These considerations illustrate the vast perspectives and challenges of the proposed new approach to studying lipid-protein interactions with sparsely fluorinated lipids.
References
- Alfredo De Biasio, Alain Ibáñez de Opakua, Mark J. Bostock, Daniel Nietlispach, Tammo Diercks and Francisco J. Blanco (2018) A generalized approach for NMR studies of lipid-protein interactions based on sparse fluorination of acyl chains Chemical Communications doi: 10.1039/C8CC02483A ↩