Remarkable enantiospecific response in Cross-Polarization Solid-State NMR experiments
If a nucleus has a non-zero spin, it behaves as a small magnet. Therefore, in an external magnetic field, the nuclear magnetic moment vector precesses about the field direction but only certain orientations are allowed by quantum rules. Thus, for hydrogen (spin 1/2) there are two possible states in the presence of a field, each with a slightly different energy. Nuclear magnetic resonance (NMR) is the absorption of radiation at a photon energy equal to the difference between these levels, causing a transition from a lower to a higher energy state.
The main application of NMR is as a technique for chemical analysis and structure determination, known as NMR spectroscopy. It depends on the fact that the electrons in a molecule shield the nucleus to some extent from the field, causing different atoms to absorb at slightly different frequencies (or at slightly different fields for a fixed frequency).
Conventionally, NMR-based techniques are considered to be intrinsically insensitive to structural differences in pure samples of the two enantiomers of the same optically-active material. This assumption, which is claimed to be valid for both liquid and solid state samples, stems directly from the idea that the chemical environment of the active magnetic nuclei in the two enantiomers is the same, and consequently magnetic resonance experiments cannot differentiate pure samples of enantiomers.
However, a striking departure from this behaviour was observed recently by a team of researchers, where an enantiospecific distinct response of a family of chiral aminoacids was documented in a series of ) experiments involving the polarization transfer from 1H to 15N nuclei. Specifically, the NMR signal intensities of the 15N nuclei for the two enantiomers of the same aminoacid were systematically larger for the D-isomer as compared to the L-isomer, while their chemical shifts remained invariant.
The researchers attributed this striking result to the onset of electron spin polarization, accompanying bond charge polarization through a chiral centre, a secondary mechanism for polarization transfer that is triggered only in the cross-polarization experimental setup. Electron spin polarization is due to the chiral-induced spin selectivity (CISS) effect, which would create an enantioselective response, analogous to the one involved in molecular recognition and enantiospecific separation with achiral magnetic substrates. This polarization influences the molecular magnetic environment, modifying the longitudinal relaxation time of 1H, and ultimately provoking the observed asymmetry in the enantiomeric response.
CISS has been found to be responsible for a number of remarkable electron spin polarization phenomena in a variety of very different experimental settings. In all of them, it was established that the observed electron spin polarization is triggered by either an electron transfer reaction or by an electron transport process through a spatially extended helical chiral structure. The new enantiospecific response detected in Cross-Polarization Magic-Angle-Spinning Solid-State NMR measurements of the 15N signal’s intensity in chiral aminoacids constituted the first reported example of the CISS effect induced by chemical bond polarization through a single chiral centre.
These findings challenge some widely accepted ideas about how to use NMR-based techniques to obtain enantiospecific response of pure materials. The team understood that new experiments were needed to further validate their theoretical model.
In the first set of experiments, the low natural abundance of 15N nuclei prevented recording 15N NMR signals without CP, i.e. in a direct pulse (DP) experiment. Now, the researchers report 1 on the results of such experiments carried out using two pairs of chiral and enantiopure molecular systems using 13C – the relatively large natural abundance of 13C nuclei makes measurements of their NMR signals more reliable. On the one hand, the organic ligands D- and L-tartaric acids and, on the other hand, 3D metal-organic frameworks (MOFs, high dimensionality nanostructured materials where organic molecules intercalated within a complex structure involving metal atoms yield versatile materials) based on the mentioned ligands and Y(III) cations.
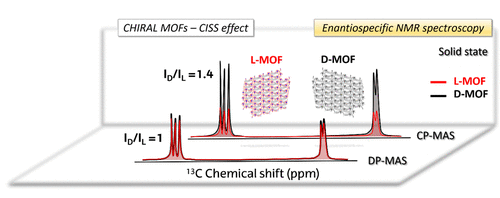
The findings reported by the team are more related to the existence of stereogenic centres in the ligands than to the extended spatial helicity that has been mentioned so far in the literature as related to the CISS effect, which indicates that the effect survives regardless of the dimensionality of the chiral object.
The samples were subjected to solid state NMR measurements, and the corresponding 13C NMR signal intensities were compared and found to be statistically different for each pair of enantiomers. Measurements in the two pairs of samples revealed that, as predicted by the theory, under DP conditions signal intensities are not enantiospecific, a conclusive result.
Thus, the theoretical model to explain the connection between the CISS effect and the remarkable enantiospecific response in Cross-Polarization Solid-State NMR experiments, this time with a 3D chiral and helical material, has been improved. The consistency of these results provides additional confidence and support to explore the implications of these experiments in the context of spin-½ transfer at interfaces, and the use of Dynamic Nuclear Polarization techniques in quantum information and quantum storage applications.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance.
Disclaimer: Parts of this article might have been copied verbatim or almost verbatim from the referenced research paper.
References
- Eider San Sebastian, Javier Cepeda, Uxua Huizi-Rayo, Alessio Terenzi, Daniel Finkelstein-Shapiro, Daniel Padro, Jose Ignacio Santos, Jon M. Matxain, Jesus M. Ugalde, and Vladimiro Mujica (2020) Enantiospecific Response in Cross-Polarization Solid-State Nuclear Magnetic Resonance of Optically Active Metal Organic Frameworks J. Am. Chem. Soc. doi: 10.1021/jacs.0c04537 ↩