Ideal furan and thiophene nanothreads
Nanothreads are one-dimensional nanomaterials composed of a primarily sp3 hydrocarbon backbone, typically formed through the compression of small molecules to high pressures. In 2015, it was found that the slow room-temperature compression of benzene produced crystalline, one-dimensional polymers composed of diamond-like bonds. These diamondoid materials that border nanotubes and polymers are predicted to possess enhanced mechanical, thermal, and chemical stability, making them ideal candidates for a diverse range of applications.
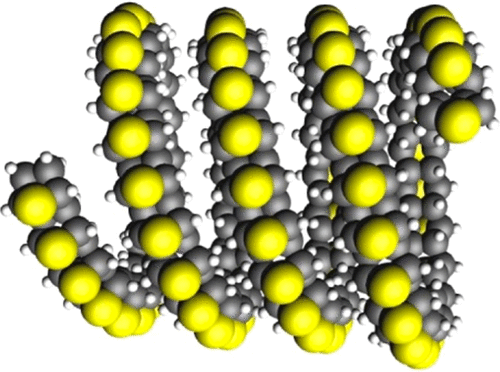
A defining feature of nanothreads is their unique combination of extreme thinness (only a few angstroms in diameter) and rigidity (multiple single covalent bonds connecting each unit). This feature distinguishes nanothreads both from traditional polymers, which are generally flexible (by rotation around single bonds), and from nanotubes or nanowires, which are normally much thicker.
Rigidity is a defining property of hard (not soft) condensed matter; when accompanied by periodicity (for which it is a precondition), it is intimately associated with a manifold of
condensed-phase properties.
Nanothreads were first synthesized from benzene under pressure in diamond anvil cells or Paris−Edinburgh cells, but the high-pressure solid-state synthesis technique appears to be quite general for unsaturated hydrocarbons. Whereas previous high-pressure reactions of small molecules resulted in amorphous products, the discovery of ordered nanothreads hints at the possibility of rational design principles for the controlled formation of crystalline extended solids at high pressure. Thus, combining physical rigidity with chemical kinetic control, one could design nanothread precursors that capture and recover molecular orbital alignments, so long as the ligands in question can be anchored to the thread backbone.
Interestingly, when precursors contain a heteroatom, as in two aromatics similar in structure, furan (a ring consisting of four CH2 groups and one oxygen atom) and thiophene (a ring of four CH2 groups and one sulphur atom), the nanothreads show enhanced structural order due, in large part, to their localized bonding. Furan and thiophene were shown to form nanothreads at 15 and 35 Gpa, respectively. Now, a team of researchers theoretically examines 1 the formation, structure, and stability of these two nanothreads, the most ordered ones produced yet.
The researchers examine three main themes. First, understanding the 20 GPa difference in the synthetic pressure (both the onset and maximum pressures) in the two nanothread syntheses. They find that the difference is explainable in terms of the greater loss of aromaticity by the thiophene.
Next, they focus on the effect of pressure on these cycloadditions using the recently developed Cammi’s extreme pressure polarizable continuum model (XP-PCM) methodology. The computed high-pressure reaction profiles of five representative reactions of the [4 + 2] polymerization show that pressure decreases the reaction barriers of all these cycloadditions due to the volume reduction nature of these bond-forming cycloadditions.
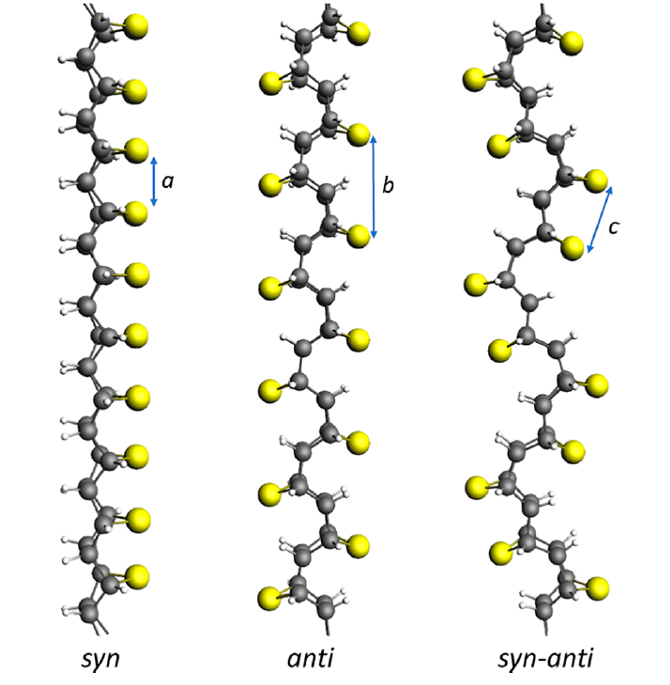
Finally, the team goes on to rationalize the structures and relative stabilities of the syn, syn−anti, and anti furan/thiophene nanothreads formed from [4 + 2] cycloaddition pathways. The syn polymer, with all O/S atoms on the same side, if not allowed to distort, is at a high energy relative to the other two due to the O/S lone pair repulsion, understandably greater for S than for O at the 2.8/2.6 Å separation. Set free, the syn isomers curve or arch in two- or three-dimensional (helical) ways, whose energetics are traced in detail. The syn polymer can also stabilize itself by twisting into zig-zag or helical energy minima. The release of strain in a linear thread as the pressure is relaxed to 1 atm, with consequent thread curving, is a likely mechanism for the observed loss of the crystalline order in the polymer as it is returned to ambient pressure.
This work provides a reasonable understanding of the factors, both intrinsic and comparative, that govern ideal furan and thiophene nanothreads. This knowledge will inform the consideration of the possibilities for other nanothreads as well.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research paper/s.
References
- Bo Chen, Vincent H. Crespi, and Roald Hoffmann (2022) Theoretical Studies of Furan and Thiophene Nanothreads: Structures, Cycloaddition Barriers, and Activation Volumes Journal of the American Chemical Society doi: 10.1021/jacs.2c01720 ↩